Abstract
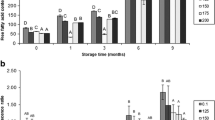
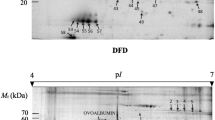
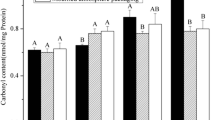
The aim of this research was to correlate the postmortem changes of sarcoplasmic proteins with instrumental color and various biochemical parameters relating to color attributes. Two-dimensional electrophoresis was used to investigate sarcoplasmic protein changes in cardiac muscle during refrigerated storage. Eleven differentially abundant protein spots were identified between storage time at day 1 and day 5 (P < 0.05). The functional category of proteins consisted of metabolic enzymes, binding proteins, chaperone proteins, and antioxidant proteins. These proteins demonstrated positive correlation with a*-value (creatine kinase M-type and malate dehydrogenase), metmyoglobin-reducing activity (aconitate hydratase, creatine kinase, and thioredoxin-dependent peroxide reductase), tissue oxygen consumption rate (creatine kinase and malate dehydrogenase), and mitochondrial respiration control ratio (thioredoxin-dependent peroxide reductase and mitochondrial superoxide dismutase). The protein patterns were likely to have impact on postmortem metabolism, thereby protecting muscle against discoloration.

Similar content being viewed by others
References
Faustman C, Cassens RG (1990) The biochemical basis for meat discoloration in fresh meat: a review. J Muscle Foods 1:217–243
Seyfert M, Mancini RA, Hunt MC, Tang J, Faustman C, Garcia M (2006) Color stability, reducing activity, and cytochrome c oxidase activity of five bovine muscles. J Agric Food Chem 54:8919–8925
Tang J, Faustman C, Hoagland TA, Mancini RA, Seyfert M, Hunt MC (2005) Postmortem oxygen consumption by mitochondria and its effects on myoglobin form and stability. J Agric Food Chem 53:1223–1230
Brown WD, Snyder HE (1969) Nonenzymatic reduction and oxidation of myoglobin and hemoglobin by nicotinamide adenine dinucleotides and flavins. J Biol Chem 244:6702–6706
Bekhit AE, Faustman C (2005) Metmyoglobin reducing activity: a review. Meat Sci 71:407–439
Tang J, Faustman C, Mancini RA, Seyfert M, Hunt MC (2005) Mitochondrial reduction of metmyoglobin: dependence on the electron transport chain. J Agric Food Chem 53:5449–5455
Sayd T, Morzel M, Chambon C, Franck M, Figwer P, Larzul C, Le Roy P, Monin G, Chérel P, Laville E (2006) Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: implications on meat color development. J Agric Food Chem 54:2732–2737
Joseph P, Suman SP, Rentfrow G, Li S, Beach CM (2012) Proteomics of muscle-specific beef color stability. J Agric Food Chem 60:3196–3203
Jia X, Ekman M, Grove H, Færgestad EM, Aass L, Hildrum KI, Hollung K (2007) Proteome changes in bovine longissimus thoracis muscle during the early postmortem storage period. J Proteome Res 6:2720–2731
Wu W, Fu Y, Therkildsen M, Li X, Dai R (2014) Molecular understanding of meat quality through application of proteomics. Food Rev Int 31:13–28
Lanari MC, Cassens RG (1991) Mitochondrial activity and beef muscle color stability. J Food Sci 56:1476–1479
Mclaren K (1987) In: McDonald R (ed) Colour space, colour scales and colour difference, edn. Dyers Company Publications Trust, Bradford, West Yorkshire, UK
Gao X, Wang Z, Miao J, Xie L, Dai Y, Li X, Chen Y, Luo H, Dai R (2014) Influence of different production strategies on the stability of color, oxygen consumption and metmyoglobin reducing activity of meat from Ningxia Tan sheep. Meat Sci 96:769–774
Smith AL (1967) Preparation, properties, and conditions for assay of mitochondria: slaughterhouse material, small-scale. Method Enzymol 10:81–86
Estabrook RW (1967) Mitochondrial respiratory control and the polarographic measurement of ADP: O ratios. Methods Enzymol 10:41–47
Mikkelsen A, Juncher D, Skibsted LH (1999) Metmyoglobin reductase activity in porcine m. longissimus dorsi muscle. Meat Sci 51:155–161
Kim YHB, Frandsen M, Rosenvold K (2011) Effect of ageing prior to freezing on colour stability of ovine longissimus muscle. Meat Sci 88:332–337
Hopkins DL, Lamb TA, Kerr MJ, van de Ven RJ, Ponnampalam EN (2013) Examination of the effect of ageing and temperature at rigor on colour stability of lamb meat. Meat Sci 95:311–316
King DA, Shackelford SD, Rodriguez AB, Wheeler TL (2011) Effect of time of measurement on the relationship between metmyoglobin reducing activity and oxygen consumption to instrumental measures of beef longissimus color stability. Meat Sci 87:26–32
Renerre M, Labas R (1987) Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Sci 19:151–165
Madhavi DL, Carpenter CH (1993) Aging and processing affect color, metmyoglobin reductase and oxygen consumption of beef muscles. J Food Sci 58:939–942
O’Keeffe M, Hood DE (1982) Biochemical factors influencing metmyoglobin formation on beef from muscles of differing colour stability. Meat Sci 7:209–228
Ostermann J, Horwich AL, Neupert W, Hartl FU (1989) Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature 341:125–130
Kim YH, Hunt MC, Mancini RA, Seyfert M, Loughin TM, Kropf DH, Smith JS (2006) Mechanism for lactate-color stabilization in injection-enhanced beef. J Agric Food Chem 54:7856–7862
Subramanian A, Miller DM (2000) Structural Analysis of α-Enolase: mapping the functional domains involved in down-regulation of the c-myc protooncogene. J Biol Chem 275:5958–5965
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. W H Freeman, New York
Garmier M, Carroll AJ, Delannoy E, Vallet C, Day DA, Small ID, Millar AH (2008) Complex I dysfunction redirects cellular and mitochondrial metabolism in arabidopsis. Plant Physiol 148:1324–1341
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochimica et Biophysica Acta (BBA) Mol Basis Dis 1762:164–180
Korzeniewski B, Zoladz JA (2004) Factors determining the oxygen consumption rate (VO2) on-kinetics in skeletal muscles. Biochem J 379:703–710
Nikawa T, Ikemoto M, Sakai T, Kano M, Kitano T, Kawahara T, Teshima S, Rokutan K, Kishi K (2002) Effects of a soy protein diet on exercise-induced muscle protein catabolism in rats. Nutrition 18:490–495
Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA (2002) Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med 33:562–571
Chae HZ, Chung SJ, Rhee SG (1994) Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem 269:27670–27678
Chan KM, Decker EA, Feustman C (1994) Endogenous skeletal muscle antioxidants. Crit Rev Food Sci 34:403–426
Nohl H, Breuninger V, Hegner D (1978) Influence of mitochondrial radical formation on energy-linked respiration. Eur J Biochem 90:385–390
Choi YM, Ryu YC, Lee SH, Go GW, Shin HG, Kim KH, Rhee MS, Kim BC (2008) Effects of supercritical carbon dioxide treatment for sterilization purpose on meat quality of porcine longissimus dorsi muscle. LWT Food Sci Technol 41:317–322
Ramanathan R, Mancini RA, Konda MR (2009) Effects of lactate on beef heart mitochondrial oxygen consumption and muscle darkening. J Agric Food Chem 57:1550–1555
Tsvetkov T, Tsonev L, Meranzov N, Minkov I (1985) Functional changes in mitochondrial properties as a result of their membrane cryodestruction: I. Influence of freezing and thawing on succinate-ferricyanide reductase of intact liver mitochondria. Cryobiology 22:47–54
Lass A, Sohal RS (1998) Electron transport-linked ubiquinone-dependent recycling of α-tocopherol inhibits autooxidation of mitochondrial membranes. Arch Biochem Biophys 352:229–236
Gatellier P, Mercier Y, Juin H, Renerre M (2005) Effect of finishing mode (pasture- or mixed-diet) on lipid composition, colour stability and lipid oxidation in meat from Charolais cattle. Meat Sci 69:175–186
Faustman C, Sun Q, Mancini R, Suman SP (2010) Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci 86:86–94
Hernández P, Zomeño L, Ariño B, Blasco A (2004) Antioxidant, lipolytic and proteolytic enzyme activities in pork meat from different genotypes. Meat Sci 66:525–529
Acknowledgments
We gratefully acknowledge the financial support by Nature Science Foundation of Hebei province China (Project No: C2015208052), Science and technology research project of Hebei Education Department (Project No: QN2015120) and the Ministry of Agriculture of China (China Agriculture Research System-39, CARS-39).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with Ethics Requirements
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Gao, X., Wu, W., Ma, C. et al. Postmortem changes in sarcoplasmic proteins associated with color stability in lamb muscle analyzed by proteomics. Eur Food Res Technol 242, 527–535 (2016). https://doi.org/10.1007/s00217-015-2563-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2563-2