Abstract
Recent reports of the widespread occurrence of the neurotoxin β-N-methylamino-l-alanine (BMAA) in cyanobacteria and particularly seafood have raised concerns for public health. LC–MS/MS is currently the analytical method of choice for BMAA determinations but incomplete separation of isomeric and isobaric compounds, matrix suppression and conjugated forms are plausible limitations. In this study, capillary electrophoresis (CE) coupled with MS/MS has been developed as an alternative method for the quantitative determination of free BMAA. Using a bare fused silica capillary, a phosphate buffer (250 mM, pH 3.0) and UV detection, it was possible to separate BMAA from four isomers, but the limit of detection (LOD) of 0.25 μg mL−1 proved insufficient for analysis of typical samples. Coupling the CE to a triple quadrupole MS was accomplished using a custom sheath-flow interface. The best separation was achieved with a 5 M formic acid in water/acetonitrile (9:1) background electrolyte. Strong acid hydrolysis of lyophilized samples was used to release BMAA from conjugated forms. Field-amplified stacking after injection was achieved by lowering sample ionic strength with a cation-exchange cleanup procedure. Quantitation was accomplished using isotope dilution with deuterium-labelled BMAA as internal standard. An LOD for BMAA in solution of 0.8 ng mL−1 was attained, which was equivalent to 16 ng g−1 dry mass in samples using the specified extraction procedure. This was comparable with LC–MS/MS methods. The method displayed excellent resolution of amino acid isomers and had no interference from matrix components. The presence of BMAA in cycad, mussel and lobster samples was confirmed by CE–MS/MS, but not in an in-house cyanobacterial reference material, with quantitative results agreeing with those from LC–MS/MS.

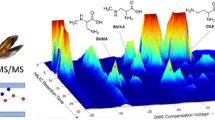
CE-MS separation and detection of BMAA, its isomers and the internal standard BMAA-d3








Similar content being viewed by others
References
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, et al. Guam amyotrophic lateral sclerosis-parkinsonism dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–22.
Bradley WG, Mash DC. Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph Lateral Scler. 2009;10 Suppl 2:7–20.
Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, et al. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand. 2009;120:216–25.
Chiu AS, Gehringer MM, Welch JH, Neilan BA. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int J Environ Res Public Health. 2011;8:3728–46.
Borenstein AR, Mortimer JA, Schofield E, Wu Y, Salmon DP, Gamst A, et al. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–71.
Vega A, Bell EA. α-Amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry. 1967;6:759–62.
Banack SA, Johnson HE, Cheng R, Cox PA. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar Drugs. 2007;5:180–96.
Marler TE, Snyder LR, Shaw CA. Cycas micronesica (Cycadales) plants devoid of endophytic cyanobacteria increase in β-methylamino-L-alanine. Toxicon. 2010;56:563–8.
Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, et al. Diverse taxa of cyanobacteria produce b-N-methylamino-L-alanine, a neurotoxic amino acid. Proc Natl Acad Sci U S A. 2005;102:5074–8.
Esterhuizen M, Downing TG. β-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol Environ Saf. 2008;71:309–13.
Johnson HE, King SR, Banack SA, Webster C, Callanaupa WJ, Cox PA. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J Ethnopharmacol. 2008;118:159–65.
Metcalf JS, Banack SA, Lindsay J, Morrison LF, Cox PA, Codd GA. Co-occurrence of β-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ Microbiol. 2008;10:702–8.
Ince PG, Codd GA. Return of the cycad hypothesis - does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol Appl Neurobiol. 2005;31:345–53.
Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology. 2002;58:956–9.
Réveillon D, Séchet V, Hess P, Amzil Z. Systematic detection of BMAA (β-N-methylamino-l-alanine) and DAB (2,4-diaminobutyric acid) in mollusks collected in shellfish production areas along the French coasts. Toxicon. 2016;110:35–46.
Réveillon D, Abadie E, Séchet V, Brient L, Savar V, Bardouil M, et al. Beta-N-methylamino-L-alanine: LC–MS/MS optimization, screening of cyanobacterial strains and occurrence in shellfish from Thau, a French Mediterranean Lagoon. Mar Drugs. 2014;12:5441–67.
Rosen J, Hellenaes K-E. Determination of the neurotoxin BMAA (β-N-methylamino-l-alanine) in cycad seed and cyanobacteria by LC–MS/MS (liquid chromatography tandem mass spectrometry). Analyst. 2008;133:1785–9.
Krüger T, Oelmüller R, Luckas B. The origin of β-N-methylamino-L-alanine (BMAA): Cycads and/-or cyanobacteria? J Endocyt Cell Res. 2012;22:29–36.
Faassen EJ. Presence of the neurotoxin BMAA in aquatic ecosystems: what do we really know? Toxins. 2014;6:1109–38.
Jiang L, Aigret B, De Borggraeve WM, Spacil Z, Ilag LL. Selective LC–MS/MS method for the identification of BMAA from its isomers in biological samples. Anal Bioanal Chem. 2012;403:1719–30.
Li A, Fan H, Ma F, McCarron P, Thomas K, Tang X, et al. Elucidation of matrix effects and performance of solid-phase extraction for LC–MS/MS analysis of β-N-methylamino-l-alanine (BMAA) and 2,4-diaminobutyric acid (DAB) neurotoxins in cyanobacteria. Analyst. 2012;137:1210–9.
Rosén J, Westerberg E, Schmiedt S, Hellenäs KE. BMAA detected as neither free nor protein bound amino acid in blue mussels. Toxicon. 2016;109:45–50.
Glover WB, Liberto CM, McNeil WS, Banack SA, Shipley PR, Murch SJ. Reactivity of β-methylamino-L-alanine in complex sample matrixes complicating detection and quantification by mass spectrometry. Anal Chem. 2012;84:7946–53.
Eriksson J, Jonasson S, Papaefthimiou D, Rasmussen U, Bergman B. Improving derivatization efficiency of BMAA utilizing AccQ-Tag in a complex cyanobacterial matrix. Amino Acids. 2009;36:43–8.
Krüger T, Moench B, Oppenhaeuser S, Luckas B. LC–MS/MS determination of the isomeric neurotoxins BMAA (β-N-methylamino-L-alanine) and DAB (2,4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revoluta and Lathyrus latifolius. Toxicon. 2010;55:547–57.
Pan M, Mabry TJ, Cao P, Moini M. Identification of nonprotein amino acids from cycad seeds as N-ethoxycarbonyl ethyl ester derivatives by positive chemical-ionization gas chromatography–mass spectrometry. J Chromatogr A. 1997;787:288–94.
Snyder LR, Hoggard JC, Montine TJ, Synovec RE. Development and application of a comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry method for the analysis of L-β-methylamino-alanine in human tissue. J Chromatogr A. 2010;1217:4639–47.
Roy-Lachapelle A, Solliec M, Sauvé S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry. Anal Bioanal Chem. 2015;407:5487–501.
Spacil Z, Eriksson J, Jonasson S, Rasmussen U, Ilag LL, Bergman B. Analytical protocol for identification of BMAA and DAB in biological samples. Analyst. 2010;135:127–32.
Banack SA, Metcalf JS, Spacil Z, Downing TG, Downing S, Long A, et al. Distinguishing the cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) from other diamino acids. Toxicon. 2011;57:730–8.
McCarron P, Logan AC, Giddings SD, Quilliam MA. Analysis of β-N-methylamino-L-alanine (BMAA) in spirulina-containing supplements by liquid chromatography-tandem mass spectrometry. Aquat Biosyst. 2014;10(5):1–7.
Combes A, El Abdellaoui S, Sarazin C, Vial J, Mejean A, Ploux O, et al. Validation of the analytical procedure for the determination of the neurotoxin β-N-methylamino-l-alanine in complex environmental samples. Anal Chim Acta. 2013;771:42–9.
Beach DG, Kerrin ES, Quilliam MA. Selective quantitation of the neurotoxin BMAA by use of hydrophilic-interaction liquid chromatography–differential mobility spectrometry–tandem mass spectrometry (HILIC–DMS–MS/MS). Anal Bioanal Chem. 2015;407:8397–409.
Jeong JS, Kim SK, Park SR. Capillary electrophoresis mass spectrometry with sheathless electrospray ionization for high sensitivity analysis of underivatized amino acids. Electrophoresis. 2012;33:2112–21.
Okafo GN, Camilleri P. Direct chiral resolution of amino acid derivatives by capillary electrophoresis. J Microcol. 1993;5:149–53.
Baptista MS, Cianca RCC, Lopes VR, Almeida CMR, Vasconcelos VM. Determination of the non protein amino acid β-N-methylamino-L-alanine in estuarine cyanobacteria by capillary electrophoresis. Toxicon. 2011;58:410–4.
Jiang L, Johnston E, Aaberg KM, Nilsson U, Ilag LL. Strategy for quantifying trace levels of BMAA in cyanobacteria by LC/MS/MS. Anal Bioanal Chem. 2013;405:1283–92.
Burton IW, Quilliam MA, Walter JA. Quantitative 1H NMR with external standards: use in preparation of calibration solutions for algal toxins and other natural products. Anal Chem. 2005;77:3123–31.
Hollingdale C, Thomas K, Lewis N, Békri K, McCarron P, Quilliam MA. Feasibility study on production of a matrix reference material for cyanobacterial toxins. Anal Bioanal Chem. 2015;407:5353–63.
Liu CC, Alary JF, Vollmerhaus P, Kadkhodayan M. Design, optimisation, and evaluation of a sheath flow interface for automated capillary electrophoresis-electrospray-mass spectrometry. Electrophoresis. 2005;26:1366–75.
Réveillon D, Abadie E, Séchet V, Masseret E, Hess P, Amzil Z. β-N-methylamino-l-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon, French Mediterranean Sea. Mar Environ Res. 2015;110:8–18.
Nunn PB, O'Brien P. The interaction of β-N-methylamino-L-alanine with bicarbonate: a proton NMR study. FEBS Lett. 1989;251:31–5.
Salomonsson ML, Fredriksson E, Alfjorden A, Hedeland M, Bondesson U. Seafood sold in Sweden contains BMAA: a study of free and total concentrations with UHPLC–MS/MS and dansyl chloride derivatization. Toxicol Rep. 2015;2:1473–81.
Bonvin G, Schappler J, Rudaz S. Capillary electrophoresis-electrospray ionization-mass spectrometry interfaces: fundamental concepts and technical developments. J Chromatogr A. 2012;1267:17–31.
Pantůčková P, Gebauer P, Boček P, Křivánková L. Recent advances in CE–MS: synergy of wet chemistry and instrumentation innovations. Electrophoresis. 2011;32:43–51.
Foret F, Thompson TJ, Vouros P, Karger BL, Gebauer P, Bocek P. Liquid sheath effects on the separation of proteins in capillary electrophoresis/electrospray mass spectrometry. Anal Chem. 1994;66:4450–8.
Sheppard RL, Tong X, Cai J, Henion JD. Chiral separation and detection of terbutaline and ephedrine by capillary electrophoresis coupled with ion spray mass spectrometry. Anal Chem. 1995;67:2054–8.
Nilsson SL, Andersson C, Sjoberg PJR, Bylund D, Petersson P, Jornten Karlsson M, et al. Phosphate buffers in capillary electrophoresis/mass spectrometry using atmospheric pressure photoionization and electrospray ionization. Rapid Comm Mass Spectrom. 2003;17:2267–72.
Stutz H, Bordin G, Rodriguez AR. Capillary zone electrophoresis of metal-binding proteins in formic acid with UV- and mass spectrometric detection using cationic transient capillary isotachophoresis for preconcentration. Electrophoresis. 2004;25:1071–89.
Ross GA. Capillary electrophoresis mass spectrometry: practical implementation and applications. LC-GC Eur. 2001;14(1):45–69.
McCormick RM. Capillary zone electrophoretic separation of peptides and proteins using low pH buffers in modified silica capillaries. Anal Chem. 1988;60:2322–8.
Soga T, Neiger DN. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem. 2000;72:1236–41.
Smith RD, Barinaga CJ, Udseth HR. Improved electrospray ionization interface for capillary zone electrophoresis-mass spectrometry. Anal Chem. 1988;60:1948–52.
Lian DS, Zhao SJ, Li J, Li BL. Progress in stacking techniques based on field amplification of capillary electrophoresis. Anal Bioanal Chem. 2014;406:6129–50.
Banack SA, Metcalf JS, Bradley WG, Cox PA. Detection of cyanobacterial neurotoxin beta-N-methylamino-l-alanine within shellfish in the diet of an ALS patient in Florida. Toxicon. 2014;90:167–73.
Acknowledgements
Technical assistance from Krista Thomas and Sabrina Giddings and discussions and advice from Daniel Beach were greatly appreciated. The National Research Council Canada and Dalhousie University are gratefully acknowledged for graduate student funding for EK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research did not involve human participants or animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 574 kb)
Rights and permissions
About this article
Cite this article
Kerrin, E.S., White, R.L. & Quilliam, M.A. Quantitative determination of the neurotoxin β-N-methylamino-l-alanine (BMAA) by capillary electrophoresis–tandem mass spectrometry. Anal Bioanal Chem 409, 1481–1491 (2017). https://doi.org/10.1007/s00216-016-0091-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0091-y