Abstract
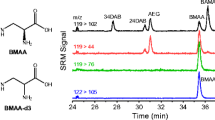
The cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) is an amino acid that is putatively associated with the pathology of amyotrophic lateral sclerosis/Parkinsonism–dementia complex (ALS-PDC) disease. It raises serious health risk concerns since cyanobacteria are ubiquitous thus making human exposure almost inevitable. The identification and quantification of BMAA in cyanobacteria is challenging because it is present only in trace amounts and occurs alongside structurally similar compounds such as BMAA isomers. This work describes an enhanced liquid chromatography/tandem mass spectrometry platform that can distinguish BMAA from its isomers β-amino-N-methyl-alanine, N-(2-aminoethyl) glycine (AEG), and 2,4-diaminobutyric acid, thus ensuring confident identification of BMAA. The method's sensitivity was improved fourfold by a post-column addition of acetonitrile. The instrument and method limits of detection were shown to be 4.2 fmol/injection (or 0.5 pg/one column) and 0.1 μg/g dry weight of cyanobacteria, respectively. The quantification method uses synthesized deuterated BMAA as an internal standard and exhibits good linearity, accuracy, and precision. Matrix effects were also investigated, revealing an ion enhancement of around 18 %. A lab-cultured cyanobacterial sample (Leptolyngbya PCC73110) was analyzed and shown to contain about 0.73 μg/g dry weight BMAA. The isomer AEG, whose chromatographic properties closely resemble those of BMAA, was also detected. These results highlight the importance of distinguishing BMAA from its isomers for reliable identification as well as providing a sensitive and accurate quantification method for measuring trace levels of BMAA in cyanobacterial samples.






Similar content being viewed by others
References
Vega A, Bell EA (1967) Alpha-amino-beta-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry 6:759
Bell EA (1964) Relevance of biochemical taxonomy to the problem of lathyrism. Nature 203:378
Kurland LT, Mulder DW (1954) Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution, with special reference to the Mariana Islands, including clinical and pathologic observations. Neurology 4:355
Mulder DW, Kurland LT (1987) Motor neuron disease: epidemiologic studies. Adv Exp Med Biol 209:325
Spencer PS, Nunn PB, Hugon J, Ludolph A, Roy DN (1986) Motorneurone disease on Guam: possible role of a food neurotoxin. Lancet 1:965
Spencer PS (1987) Guam ALS/parkinsonism-dementia: a long-latency neurotoxic disorder caused by “slow toxin(s)” in food? Can J Neurol Sci 14:347
Nunn PB, Seelig M, Zagoren JC, Spencer PS (1987) Stereospecific acute neuronotoxicity of ‘uncommon’ plant amino acids linked to human motor-system diseases. Brain Res 410:375
Ross SM, Spencer PS (1987) Specific antagonism of behavioral action of “uncommon” amino acids linked to motor system diseases. Synapse 1:248
Duncan MW, Kopin IJ, Garruto RM, Lavine L, Markey SP (1988) 2-Amino-3 (methylamino)-propionic acid in cycad derived foods is an unlikely cause of amyotrophic lateral sclerosis/parkinsonism. Lancet 2:631
Duncan MW, Steele JC, Kopin IJ, Markey SP (1990) 2-Amino-3-(methylamino)-propanoic acid (BMAA) in cycad flour: an unlikely cause of amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Neurology 40:767
Cox P, Banack S, Murch S (2003) Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci USA 100:13380
Cox PA, Sacks OW (2002) Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 58:956
Banack SA, Cox PA (2003) Biomagnification of cycad neurotoxins in flying foxes. Neurology 61:387
Murch SJ, Cox PA, Banack SA (2004) A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc Natl Acad Sci USA 101:12228
Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B (2005) Diverse taxa of cyanobacteria produce beta-N-methylamino-l-alanine, a neurotoxic amino acid. Proc Natl Acad Sci USA 102:5074
Metcalf JS, Banack SA, Lindsay J, Morrison LF, Cox PA, Codd GA (2008) Co-occurrence of beta-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ Microbiol 10(3):702–708
Faassen EJ, Gillissen F, Zweers HAJ, Luerling M (2009) Determination of the neurotoxins BMAA (beta-N-methylamino-l-alanine) and DAB (alpha-gamma-diaminobutyric acid) by LC-MSMS in Dutch urban waters with cyanobacterial blooms. Amyotroph Lateral Scler 10:79
Brand LE, Pablo J, Compton A, Hammerschlag N, Mash DC (2010) Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 9:620
Mondo K, Hammerschlag N, Basile M, Pablo J, Banack SA, Mash DC (2012) Cyanobacterial neurotoxin beta-N-methylamino alanine (BMAA) in shark fins. Mar Drugs 10:509
Esterhuizen M, Downing T (2008) beta-N-methylamino-l-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol Environ, 71(2):309–313
Esterhuizen-Londt M, Downing S, Downing TG (2011) Improved sensitivity using liquid chromatography mass spectrometry (LC-MS) for detection of propyl chloroformate derivatised beta-N-methylamino-l-alanine (BMAA) in cyanobacteria. Water SA 37:133
Jonasson S, Eriksson J, Berntzon L, Spacil Z, Ilag LL, Ronnevi L, Rasmussen U, Bergman B (2010) Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc Natl Acad Sci USA 107:9252
Bidigare RR, Christensen SJ, Wilde SB, Banack SA (2009) Cyanobacteria and BMAA: possible linkage with avian vacuolar myelinopathy (AVM) in the south-eastern United States. Amyotroph Lateral Scler 10(Suppl 2):71–73
Craighead D, Metcalf JS, Banack SA, Amgalan L, Reynolds HV, Batmunkh M (2009) Presence of the neurotoxic amino acids beta-N-methylamino-l-alanine (BMAA) and 2,4-diamino-butyric acid (DAB) in shallow springs from the Gobi Desert. Amyotroph Lateral Scler 10:96
Roney BR, Li R, Banack SA, Murch S, Honegger R, Cox PA (2009) Consumption of fa cai Nostoc soup: a potential for BMAA exposure from Nostoc cyanobacteria in China? Amyotroph Lateral Scler 10:44
Li A, Tian Z, Li J, Yu R, Banack SA, Wang Z (2010) Detection of the neurotoxin BMAA within cyanobacteria isolated from freshwater in China. Toxicon 55:947
Cox PA, Richer R, Metcalf JS, Banack SA, Codd GA, Bradley WG (2009) Cyanobacteria and BMAA exposure from desert dust: a possible link to sporadic ALS among Gulf War veterans. Amyotroph Lateral Scler 10:109
Johnson HE, King SR, Banack SA, Webster C, Callanaupa WJ, Cox PA (2008) Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J Ethnopharmacol 118:159
Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW (2004) Occurrence of beta-methylamino-L-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand 110:267
Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, Buck A, Mash DC (2009) Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand 120:216
Rosen J, Hellenaes K (2008) Determination of the neurotoxin BMAA (beta-N-methylamino-l-alanine) in cycad seed and cyanobacteria by LC-MS/MS (liquid chromatography tandem mass spectrometry). Analyst 133:1785 (Cambridge, UK)
Banack SA, Metcalf JS, Spacil Z, Downing TG, Downing S, Long A, Nunn PB, Cox PA (2011) Distinguishing the cyanobacterial neurotoxin beta-N-methylamino-l-alanine (BMAA) from other diamino acids. Toxicon 57:730
Snyder LR, Hoggard JC, Montine TJ, Synovec RE (2010) Development and application of a comprehensive two dimensional gas chromatography with time-of-flight mass spectrometry method for the analysis of L-beta-methylamino-alanine in human tissue. J Chromatogr A 1217:4639
Spacil Z, Eriksson J, Jonasson S, Rasmussen U, Ilag LL, Bergman B (2010) Analytical protocol for identification of BMAA and DAB in biological samples. Analyst 135:127
Jiang L, Aigret B, De Borggraeve WM, Spacil Z, Ilag LL (2012) Selective LC-MS/MS method for the identification of BMAA from its isomers in biological samples. Anal Bioanal Chem 403:1719
Snyder LR, Cruz-Aguado R, Sadilek M, Galasko D, Shaw CA, Montine TJ (2009) Parkinson-dementia complex and development of a new stable isotope dilution assay for BMAA detection in tissue. Toxicol Appl Pharmacol 240:180
Kruger T, Monch B, Oppenhauser S, Luckas B (2010) LC-MS/MS determination of the isomeric neurotoxins BMAA (beta-N-methylamino-l-alanine) and DAB (2,4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revoluta and Lathyrus latifolius. Toxicon 55:547
Zhou S, Hamburger M (1995) Effects of solvent composition on molecular ion response in electrospray mass spectrometry: investigation of the ionization processes. Rapid Commun Mass Spectrom 9:1516
Iribarne JV, Dziedzic PJ, Thomson BA (1983) Atmospheric pressure ion evaporation-mass spectrometry. Int J Mass Spectrom Ion Phys 50:331
Glover WB, Liberto CM, McNeil WS, Banack SA, Shipley PR, Murch SJ (2012) Reactivity of beta-methylamino-l-alanine in complex sample matrixes complicating detection and quantification by mass spectrometry. Anal Chem 84:7946 (Washington, DC, US)
Faassen EJ, Gillissen F, Luerling M (2012) A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS One 7:36667
Taylor PJ (2005) Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray tandem mass spectrometry. Clin Biochem 38:328
Li A, Fan H, Ma F, McCarron P, Thomas K, Tang X, Quilliam MA (2012) Elucidation of matrix effects and performance of solid-phase extraction for LC-MS/MS analysis of beta-N-methylamino-l-alanine (BMAA) and 2,4-diaminobutyric acid (DAB) neurotoxins in cyanobacteria. Analyst 137:1210 (Cambridge, UK)
Banack SA, Metcalf JS, Jiang L, Craighead D, Ilag LL, Cox PA (2012) Cyanobacteria produce N-(2-aminoethyl)glycine, a backbone for peptide nucleic acids which may have been the first genetic molecules for life on earth. PLoS One 7:e49043
Acknowledgments
We thank Prof. Birgitta Bergman and Dr. Johan Eriksson (Department of Botany, Stockholm University) for providing the samples used in this study.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 77 kb)
Rights and permissions
About this article
Cite this article
Jiang, L., Johnston, E., Åberg, K.M. et al. Strategy for quantifying trace levels of BMAA in cyanobacteria by LC/MS/MS. Anal Bioanal Chem 405, 1283–1292 (2013). https://doi.org/10.1007/s00216-012-6550-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6550-1