Abstract
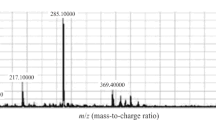
Cannabis is not only a widely used illicit drug but also a substance which can be used in pharmacological therapy because of its analgesic, antiemetic, and antispasmodic properties. A very rapid and sensitive method for determination of ∆9-tetrahydrocannabinol (THC), the principal active component of cannabis, and two of its phase I metabolites in plasma has been developed and validated. After solid-phase extraction of plasma (0.2 mL), the clean extracts were analyzed by tandem mass spectrometry after a 5-min liquid chromatographic separation. The linear calibration ranges were from 0.05 to 30 ng mL−1 for THC and 11-nor-∆9-carboxy-tetrahydrocannabinol (THC-COOH) and from 0.2 to 30 ng mL−1 for ∆9-(11-OH)-tetrahydrocannabinol (11-OH-THC). Imprecision and inaccuracy were always below 7 and 12 % (expressed as relative standard deviation and relative error), respectively. The method has been successfully applied to determination of the three analytes in plasma obtained from healthy volunteers after oral administration of 20 mg dronabinol.


Similar content being viewed by others
References
EMCDDA (2011) The State of the Drugs Problem in Europe, Annual Report 2011. European Monitoring Centre for Drugs and Drug Addiction
Hall W, Degenhardt L (2009) Adverse health effects of non-medical cannabis use. Lancet 374:1383–1391
Seely KA, Prather PL, James LP, Moran JH (2011) Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv 11:36–51
Husseini L, Leussink VI, Warnke C, Hartung HP, Kieseier BC (2011) Cannabinoids for symptomatic therapy of multiple sclerosis. Nervenarzt. doi:10.1007/s00115-011-3401-9
Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I (2007) Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci 80:1415–1419
Watanabe K, Matsunaga T, Yamamoto I, Funae Y, Yoshimura H (1995) Involvement of CYP2C in the metabolism of cannabinoids by human hepatic microsomes from an old woman. Biol Pharm Bull 18:1138–1141
Richardson TH, Jung F, Griffin KJ, Wester M, Raucy JL, Kemper B, Bornheim LM, Hassett C, Omiecinski CJ, Johnson EF (1995) A universal approach to the expression of human and rabbit cytochrome P450s of the 2C subfamily in Escherichia coli. Arch Biochem Biophys 323:87–96
Schwope DM, Scheidweiler KB, Huestis MA (2011) Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 401:1273–1283
Vree TB, Breimer DD, van Ginneken CA, van Rossum JM, de Zeeuw RA, Witte AH (1971) Identification of cannabivarins in hashish by a new method of combined gas chromatography–mass-spectrometry. Clin Chim Acta 34:365–372
Mikes F, Hofmann A, Waser PG (1971) Identification of (−)-delta 9-6a,10a-trans-tetrahydrocannabinol and two of its metabolites in rats by use of combination gas chromatography–mass spectrometry and mass fragmentography. Biochem Pharmacol 20:2469–2476
Breindahl T, Andreasen K (1999) Determination of 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in urine using high-performance liquid chromatography and electrospray ionization mass spectrometry. J Chromatogr B: Biomed Sci Appl 732:155–164
Backstrom B, Cole MD, Carrott MJ, Jones DC, Davidson G, Coleman K (1997) A preliminary study of the analysis of cannabis by supercritical fluid chromatography with atmospheric pressure chemical ionisation mass spectroscopic detection. Sci Justice 37:91–97
Elian AA, Hackett J (2009) Solid-phase extraction and analysis of THC and carboxy-THC from whole blood using a novel fluorinated solid-phase extraction sorbent and fast liquid chromatography–tandem mass spectrometry. J Anal Toxicol 33:461–468
del Mar Ramirez Fernandez M, de Boeck G, Wood M, Lopez-Rivadulla M, Samyn N (2008) Simultaneous analysis of THC and its metabolites in blood using liquid chromatography–tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 875:465–470
Valiveti S, Hammell DC, Earles DC, Stinchcomb AL (2005) LC–MS method for the estimation of delta8-THC and 11-nor-delta8-THC-9-COOH in plasma. J Pharm Biomed Anal 38:112–118
de Jager AD, Bailey NL (2011) Online extraction LC-MS/MS method for the simultaneous quantitative confirmation of urine drugs of abuse and metabolites: amphetamines, opiates, cocaine, cannabis, benzodiazepines and methadone. J Chromatogr B Anal Technol Biomed Life Sci 879:2642–2652
Grauwiler SB, Scholer A, Drewe J (2007) Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-plasma and urine after small doses of Cannabis sativa extracts. J Chromatogr B Anal Technol Biomed Life Sci 850:515–522
Gray TR, Barnes AJ, Huestis MA (2010) Effect of hydrolysis on identifying prenatal cannabis exposure. Anal Bioanal Chem 397:2335–2347
Konig S, Aebi B, Lanz S, Gasser M, Weinmann W (2011) On-line SPE LC-MS/MS for the quantification of Delta9-tetrahydrocannabinol (THC) and its two major metabolites in human peripheral blood by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 400:9–16
Jagerdeo E, Montgomery MA, Karas RP, Sibum M (2010) A fast method for screening and/or quantitation of tetrahydrocannabinol and metabolites in urine by automated SPE/LC/MS/MS. Anal Bioanal Chem 398:329–338
Weinmann W, Goerner M, Vogt S, Goerke R, Pollak S (2001) Fast confirmation of 11-nor-9-carboxy-Delta(9)-tetrahydrocannabinol (THC-COOH) in urine by LC/MS/MS using negative atmospheric-pressure chemical ionisation (APCI). Forensic Sci Int 121:103–107
Weinmann W, Vogt S, Goerke R, Muller C, Bromberger A (2000) Simultaneous determination of THC-COOH and THC-COOH-glucuronide in urine samples by LC/MS/MS. Forensic Sci Int 113:381–387
Felli M, Martello S, Chiarotti M (2011) LC-MS-MS method for simultaneous determination of THCCOOH and THCCOOH-glucuronide in urine: Application to workplace confirmation tests. Forensic Sci Int 204:67–73
Chebbah C, Pozo OJ, Deventer K, Van Eenoo P, Delbeke FT (2010) Direct quantification of 11-nor-Delta(9)-tetrahydrocannabinol-9-carboxylic acid in urine by liquid chromatography/tandem mass spectrometry in relation to doping control analysis. Rapid Commun Mass Spectrom 24:1133–1141
Teixeira H, Proenca P, Castanheira A, Santos S, Lopez-Rivadulla M, Corte-Real F, Marques EP, Vieira DN (2004) Cannabis and driving: the use of LC–MS to detect delta9-tetrahydrocannabinol (delta9-THC) in oral fluid samples. Forensic Sci Int 146(Suppl):S61–S63
Molnar A, Lewis J, Doble P, Hansen G, Prolov T, Fu S (2011) A rapid and sensitive method for the identification of delta-9-tetrahydrocannabinol in oral fluid by liquid chromatography–tandem mass spectrometry. Forensic Sci Int. doi:10.1016/j.forsciint.2011.01.045:
Walter C, Oertel BG, Felden L, Nöth U, Deichmann R, Lötsch J (2011) The effects of delta-9-tetrahydrocannabinol on nasal chemosensitivity: a pharmacological fMRI study in healthy volunteers. Naunyn Schmiedebergs Arch Pharmacol 383(Suppl 1):75
Guidance for Industry: Bioanalytical Method Validation (2001) US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Rockville, USA www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf. Accessed 21 Jun 2012
Smith G (2010) Bioanalytical method validation: notable points in the 2009 draft EMA Guideline and differences with the 2001 FDA Guidance. Bioanalysis 2:929–935
Validation of Analytical Procedures: Text and Methodology Q2(R1) (2005) ICH. International Conference on Harmonisation of Technical Requirements, ICH Harmonised Tripartite Guideline, Geneva, Switzerland. http://private.ich.org/LOB/media/MEDIA417.pdf. Accessed 21 Jun 2012
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030
Wall ME, Perez-Reyes M (1981) The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. J Clin Pharmacol 21:178S–189S
Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42:327–360
Dubois N, Paccou AP, De Backer BG, Charlier CJ (2012) Validation of the quantitative determination of tetrahydrocannabinol and its two major metabolites in plasma by ultra-high-performance liquid chromatography–tandem mass spectrometry according to the total error approach. J Anal Toxicol 36:25–29
Milman G, Schwope DM, Schwilke EW, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA (2011) Oral fluid and plasma cannabinoid ratios after around-the-clock controlled oral delta(9)-tetrahydrocannabinol administration. Clin Chem 57:1597–1606
Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA (2011) Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 57:66–75
Acknowledgments
This study was supported in part by the “Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz: LOEWE. Schwerpunkt: Anwendungsorientierte Arzneimittelforschung” (GG, JL), the “Deutsche Forschungsgemeinschaft, DFG Lo 612/10-1 (JL)”, and the European Graduate School GRK757 (GG, JL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreirós, N., Labocha, S., Walter, C. et al. Simultaneous and sensitive LC–MS/MS determination of tetrahydrocannabinol and metabolites in human plasma. Anal Bioanal Chem 405, 1399–1406 (2013). https://doi.org/10.1007/s00216-012-6501-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6501-x