Abstract
Little attention has been paid so far to the influence of the chemical nature of the substance when measuring δ 15N by elemental analysis (EA)–isotope ratio mass spectrometry (IRMS). Although the bulk nitrogen isotope analysis of organic material is not to be questioned, literature from different disciplines using IRMS provides hints that the quantitative conversion of nitrate into nitrogen presents difficulties. We observed abnormal series of δ 15N values of laboratory standards and nitrates. These unexpected results were shown to be related to the tailing of the nitrogen peak of nitrate-containing compounds. A series of experiments were set up to investigate the cause of this phenomenon, using ammonium nitrate (NH4NO3) and potassium nitrate (KNO3) samples, two organic laboratory standards as well as the international secondary reference materials IAEA-N1, IAEA-N2—two ammonium sulphates [(NH4)2SO4]—and IAEA-NO-3, a potassium nitrate. In experiment 1, we used graphite and vanadium pentoxide (V2O5) as additives to observe if they could enhance the decomposition (combustion) of nitrates. In experiment 2, we tested another elemental analyser configuration including an additional section of reduced copper in order to see whether or not the tailing could originate from an incomplete reduction process. Finally, we modified several parameters of the method and observed their influence on the peak shape, δ 15N value and nitrogen content in weight percent of nitrogen of the target substances. We found the best results using mere thermal decomposition in helium, under exclusion of any oxygen. We show that the analytical procedure used for organic samples should not be used for nitrates because of their different chemical nature. We present the best performance given one set of sample introduction parameters for the analysis of nitrates, as well as for the ammonium sulphate IAEA-N1 and IAEA-N2 reference materials. We discuss these results considering the thermochemistry of the substances and the analytical technique itself. The results emphasise the difference in chemical nature of inorganic and organic samples, which necessarily involves distinct thermochemistry when analysed by EA-IRMS. Therefore, they should not be processed using the same analytical procedure. This clearly impacts on the way international secondary reference materials should be used for the calibration of organic laboratory standards.

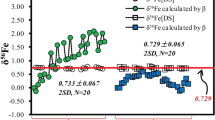
Control chart of the δ 15N value of IAEA-N1, IAEA-NO-3 and NH4NO3 analysed a) with oxygen injection (analytical cycle 70 s, oxygen for 60 s, sample start and stop at 18 s/20 s), b) with oxygen injection (analytical cycle 70 s, oxygen for 60 s, sample start and stop at 0 s/2 s and 5 s/7 s), c) without oxygen injection (analytical cycle 70 s, sample start and stop at 18 s/20 s).








Similar content being viewed by others
References
Aranda R IV, Stern LA, Dietz ME, McCormick MC, Barrow JA, Mothershead RF II (2011) Forensic utility of isotope ratio analysis of the explosive urea nitrate and its precursors. Forensic Sci Int 206(1–3):143–149. doi:10.1016/j.forsciint.2010.07.030
Benson SJ, Lennard CJ, Hill DM, Maynard P, Roux C (2010) Forensic analysis of explosives using isotope ratio mass spectrometry (IRMS)—part 1: instrument validation of the DELTA XP IRMS for bulk nitrogen isotope ratio measurements. J Forensic Sci 55(1):193–204. doi:10.1111/j.1556-4029.2009.01241.x
Benson S (2009) Introduction of isotope ratio mass spectrometry (IRMS) for the forensic analysis of explosives. University of Technology of Sydney, Sydney
Benson SJ, Lennard CJ, Maynard P, Hill DM, Andrew AS, Neal K, Stuart-Williams H, Hope J, Stewart Walker G, Roux C (2010) Forensic analysis of explosives using isotope ratio mass spectrometry (IRMS)—part 2: forensic inter-laboratory trial: bulk carbon and nitrogen stable isotopes in a range of chemical compounds (Australia and New Zealand). J Forensic Sci 55(1):205–212. doi:10.1111/j.1556-4029.2009.01242.x
Forensic Isotope Ratio Mass Spectrometry Network (2011) FIRMS newsletter Spring 2011. Forensic Isotope Ratio Mass Spectrometry. Network, Bristol
Böhlke JK, Coplen TB (1993) Interlaboratory comparison of reference materials for nitrogen isotope ratio measurements. In: 5th IAEA meeting on stable isotope standards and intercomparison materials, Vienna. IAEA-TECDOC-825. International Atomic Energy Agency, Vienna, pp 51–62
Silva SR, Kendall C, Wilkinson DH, Ziegler AC, Chang CCY, Avanzino RJ (2000) A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen isotope ratios. J Hydrol 228:22–36
Spoelstra J, Schiff SL, Jeffries DS, Semkin RG (2004) Effect of storage on the isotopic composition of nitrate in bulk precipitation. Environ Sci Technol 38(18):4723–4727
Noguchi J (1951) Improved method for quantitative nitrogen analysis of organic compounds. Sci Pap Osaka Univ 22(1):1–14
Borda P, Hayward LD (1967) Nitrogen analysis of nitrate esters by micro-Dumas combustion. Anal Chem 39(4):548–549
Schindler FV, Knighton RE (1999) Sample preparation for total nitrogen and 15N ratio analysis by the automated Dumas combustion method. Commun Soil Sci Plant 30(9–10):1315–1324
ISOGEOCHEM (2012) An e-mail discussion list and reference web site for stable isotope geochemistry. http://isogeochem.wikispaces.com/
Coplen TB (2011) Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom 25:2538–2560
Paul D, Skrzypek G, Forizs I (2007) Normalization of measured stable isotopic compositions to isotope reference scales—a review. Rapid Commun Mass Spectrom 21(18):3006–3014. doi:10.1002/rcm.3185
Taverniers I, De Loose M, Van Bockstaele E (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal Chem 23(8):535–552
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry. Pearson, Harlow
Joint Committee for Guides in Metrology (2008) Evaluation of measurement data—guide to the expression of uncertainty in measurement. JCGM 100:2008. Bureau International des Poids et des Mesures, Paris
Pella E, Colombo B (1973) Study of carbon, hydrogen and nitrogen determination by combustion-gas chromatography. Mikrochim Acta 5:697–719
Stull DR, Prophet H (1971) JANAF thermodynamical tables, 2nd edn. US National Bureau of Standards. Office of Standards Reference Materials, Washington
Burcat A, Ruscic B (2005) Third millennium ideal gas and condensed phase thermochemical database for combustion with updates from active thermochemical tables, vol ANL-05/20. Argonne technical publication. See also http://garfield.chem.elte.hu/Burcat/burcat.html
Stern KH (2001) High temperature properties and thermal decomposition of inorganic salts with oxyanions. CRC, Boca Raton
Johnston HS, Foering L, Thompson RJ (1953) Kinetics of the thermal decomposition of nitric acid vapor. II. Mechanism. J Phys Chem 57(4):390–395
Degobert P (1992) Automobile et pollution. Technip, Paris
Révész K, Böhlke JK, Yoshinari T (1997) Determination of δ 18O and δ 15N in nitrate. Anal Chem 69(21):4375–4380
Acknowledgments
The authors thank Catharina Lötscher and Matthias Saurer for their help with the numerous isotopic analyses. They also acknowledge Thomas Kuhn and Oliver Kracht from Thermo Fisher Scientific for their valuable reflections and advice on this issue. The authors are also grateful to the anonymous reviewers for their helpful comments and suggestions regarding the manuscript. This research was partly funded by the Fondation du 450ème Anniversaire de l’Université de Lausanne and the Société Académique Vaudoise.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2061 kb)
Appendix
Appendix
Tables 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 provide supplementary material for thermochemical evaluation of standard reactions (standard state: 1 atm).
Rights and permissions
About this article
Cite this article
Gentile, N., Rossi, M.J., Delémont, O. et al. δ 15N measurement of organic and inorganic substances by EA-IRMS: a speciation-dependent procedure. Anal Bioanal Chem 405, 159–176 (2013). https://doi.org/10.1007/s00216-012-6471-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6471-z