Abstract
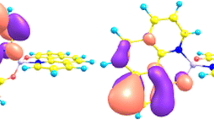
The three radical, anion and cation forms of B2N(−,0,+) have been studied inside the B12N12 ring and compared with one another in terms of non-covalent interaction. Not only does the symmetry breaking (SB) of B2N(−,0,+) exhibit an energy barrier in the scale of millihartree (10–50) (depending on whether it is in on ionic or neutral forms), but it also create several SBs through the asymmetry stretching and bending mode interactions. We have previously figured out that the double well minimum of the BNB’s potential diagram is due to the lack of the proper permutational symmetry of its wave function and charge distribution. In this study, an extreme enhancement in the energy barrier of SB effect was observed upon the interaction of BNB (both radical and cation) with B12N12 ring. Such amount was not observed for the isolated BNB structure. Depending on the diameter of B n N n and whether the system is ionic or radical, the interaction of B2N(0,−,+) with the ring is either repulsive or attractive. The BN(−,0,+) B–B12N12 system works as a quantum rotatory system, which has a range of spectrum in the IR region due to the alternative attraction and repulsion forces.







Similar content being viewed by others
Notes
It was first shown by John Lennard-Jones [26] that the temperature-averaged dipole–dipole interaction is \( \bar{E}_{{{\text{dip}} - {\text{dip}}}} = - \frac{2kT}{{3R_{AB}^{6} }}\mu_{A}^{2} \mu_{B}^{2} \), since averaged energy has R−6 dependence.
An improved method for computing potential-derived charges is described which is used upon the CHELP program available from QCPE. This approach (CHELPG) is shown to be considerably less dependent upon molecular orientation than the original CHELP program. The results are compared to those obtained by using CHELP.
In the CHELPG (charges from electrostatic potentials using a grid-based method), atomic charges are fitted to reproduce the molecular electrostatic potential (MESP) at a number of points around the molecule. The MESP is calculated at a number of grid points spaced 3.0 pm apart and distributed regularly in a cube. Charges derived in this way do not necessarily reproduce the dipole moment of the molecule. CHELPG charges are frequently considered superior to Mulliken charges as they depend much less on the underlying theoretical method used to compute the wave function (and thus the MESP).
References
Martin JML, François J-P, Gijbels R (1989) J Chem Phys 90:6469
Asmis KR, Taylor TR, Neumark DM (1999) J Chem Phys 111:8838
Martin JML, François J-P, Gijbels R (1990) Chem Phys Lett 172:354
Knight LB Jr, Hill DW, Kirk TJ, Arrington CA (1992) J Phys Chem 96:555
Martin JML, François J-P, Gijbels R (1992) Chem Phys Lett 193:243
Hassanzadeh P, Andrews L (1992) J Phys Chem 96:9177
Andrews L, Hassanzadeh P, Burkholder TR, Martin JML (1993) J Chem Phys 98:922
Thompson CA, Andrews L (1995) J Am Chem Soc 117:10125
Li X, Paldus J (2007) J Chem Phys 126:224304
Martin JML, El-Yazal J, François J-P, Gijbels R (1995) Mol Phys 85:527
Meloni G, Sai Baba M, Gingerich A (2000) J Chem Phys 113:8995
Graham WRK, Weltner W (1976) J Chem Phys 65:1516
Monajjemi M (2013) Chem Phys 425:29–45
Monajjemi M, Lee VS, Khaleghian M, Honarparvar B, Mollaamin F (2010) J Phys Chem C 114:15315
Monajjemi M, Boggs JE (2013) J Phys Chem A 117:1670
Monajjemi M (2012) Struct Chem 23:551
Walsh AD (1953) J Chem Soc 2260–2266. doi:10.1039/JR9530002260
London F (1930) Z Phys 63:245
Eisenschitz R, London F (1930) Z Phys 60:491
Physik Z (1937) Chemie 33:8–26
Szalewicz K, Jeziorski B (1995) In: Scheiner S (ed) Molecular theory of gases and liquids. Wiley, Chichester. ISBN 0-471-95921-9
Hirschfelder O, Curtiss CF, Bird RB (1954) Molecular theory of gases and liquids. Wiley, New York
Stone AJ (1996) The Theory of Intermolecular Forces. Clarendon Press, Oxford
Pyykkö P, Wang C, Straka M, Vaara J (2007) Phys Chem Chem Phys 9:2954–2958
Wang C, Pyykkö P, Straka M (2010) Phys Chem Chem Phys 12:6187–6203
Lennard-Jones JE (1931) Proc Phys Soc (London) 43:461
Edmonds AR (1957) Angular momentum in quantum mechanics. Princeton University Press, Princeton
Thompson WJ (1994) Angular momentum. Wiley, New York
Thorne KS (1980) Rev Mod Phys 52(2):299
Brink DM, Satchler GR (1968) Angular momentum, 2nd edn. Clarendon Press, Oxford
Barone V (1996) In: Chong DP (ed) Recent advances in density functional methods, Parts I. World Scientific Publ. Co
Chopra NG, Luyken RJ, Cherrey K, Crespi VH, Cohen ML, Louie SG, Zettl A (1995) Science 269:966
Blasé X, Rubio A, Louie SG, Cohen ML (1994) Europhys Lett 28L:335
Blasé X, Charlier JC, de Vita A, Car R (1997) Appl Phys Lett 70:197
Walker TEH, Richards WG (1970) J Chem Phys 52:1311
Koseki S, Schmidt MW, Gordon MS (1992) J Phys Chem 96:10768
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968
Bader RFW (1990) Atoms in molecule: a quantum theory. Oxford Univ. Press, Oxford
Besler BH, Merz KM, Kollman PA (1990) J Comput Chem 11:431
Chirlian LE, Francl MM (1987) J Comp Chem 8:894
Brneman CM, Wiberg KB (1990) J Comp Chem 11:361
Martin F, Zipse H (2005) J Comp Chem 26:97–105
Frisch MJ, Trucks GW, Schlegel HB, Scusera GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, revision A 7. Gaussian Inc, Pittsburgh
Zhao Yan, Truhlar Donald G (2008) Theor Chem Acc 120:215–241
Zhao Yan, Truhlar Donald G (2008) Acc Chem Res 41(2):157–167
Check CE, Gilbert TM (2005) J Org Chem 70:9828–9834
Grimme SS (2006) Angew Chem Int Ed 45:4460–4464
Wodrich MD, Corminboeuf C, Schleyer PVR (2006) Org Lett 8:3631–3634
Schreiner PR, Fokin AA, Pascal RA Jr, de Meijere A (2006) Org Lett 8:3635–3638
Zhao Y, Truhlar DG (2006) Org Lett 8:5753–5755
Gwaltney SR, Head-Gordon M (2001) Phys Chem Chem Phys 3:4495
Ding H, Morse MD, Apetrei C, Chacaga L, Aier JP (2006) J Chem Phys 125:194315
Liu Y, Zou W, Bersuker IB, Boggs JE (2009) J Chem Phys 130:184305
Al-Saidi WA (2012) Chem Phys Lett 543:41–44
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monajjemi, M. Non-covalent attraction of B2N(−,0) and repulsion of B2N(+) in the B n N n ring: a quantum rotatory due to an external field. Theor Chem Acc 134, 77 (2015). https://doi.org/10.1007/s00214-015-1668-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1668-9