Abstract
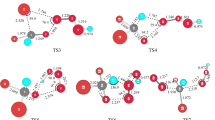
The forward and reverse reactions Br + H2O → HBr + OH are important in atmospheric and environmental chemistry. Five stationary points on the potential energy surface for the Br + H2O → HBr + OH reaction, including the entrance complex, transition state, and exit complex, have been studied using the CCSD(T) method with correlation-consistent basis sets up to cc-pV5Z-PP. Contrary to the valence isoelectronic F + H2O system, the Br + H2O reaction is endothermic (by 31.8 kcal/mol after zero-point vibrational, relativistic, and spin–orbit corrections), consistent with the experimental reaction enthalpy. The CCSD(T)/cc-pV5Z-PP method predicts that the reverse reaction HBr + HO → Br + H2O has a complex but no classical barrier. When zero-point vibrational energies are added, the transition state lies 0.25 kcal/mol above the separated products. This is consistent with the negative temperature dependence for the rate constant observed in experiments. The entrance complex is predicted to lie 2.6 kcal/mol below separated Br + H2O. The exit complex is predicted to lie 1.8 kcal/mol below separated HBr + OH.




Similar content being viewed by others
References
Sander SP, Friedl RR, Barker JR, Golden DM, Kurylo MJ, Wine PH, Abbatt J, Burkholder JB, Kolb CE, Moortgat GK, Huie RE, Orkin VL (2009) Chemical kinetics and photochemical data for use in stratospheric studies evaluation number 16. NASA, Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA
Holloway AM, Wayne RP (2010) Atmospheric chemistry. RSC Publishing, Cambridge
Clark DR, Simmons RF, Smith DA (1970) Trans Faraday Soc 66:1423–1435
Sims IR, Smith IWM, Clary DC, Bocherel P, Rowe BR (1994) J Chem Phys 101:1748–1751
Clary DC, Nyman G, Hernandez R (1994) J Chem Phys 101:3704–3714
Nizamov B, Setser DW, Wang H, Peslherbe GH, Hase WL (1996) J Chem Phys 105:9897–9911
Jaramillo VI, Gougeon S, Le Picard SD, Canosa A, Smith MA, Rowe BR (2002) Int J Chem Kinet 34:339–344
Che DC, Matsuo T, Yano Y, Bonnet L, Kasai T (2008) Phys Chem Chem Phys 10:1419–1423
Liu JY, Li ZS, Dai ZW, Huang XR, Sun CC (2001) J Phys Chem A 105:7707–7712
Tsai PY, Che DC, Nakamura M, Lin KC, Kasai T (2011) Phys Chem Chem Phys 13:1419–1423
Ravishankara AR, Wine PH, Langford AO (1979) Chem Phys Lett 63:479–484
Atkinson DB, Jaramillo VI, Snith MA (1997) J Phys Chem A 101:3356–3359
Bedjianian Y, Riffault V, Le Bras G, Poulet G (1999) J Photochem Photobiol A Chem 128:15–25
Wilson WE, O’Donovan JT, Fristrom RM (1969) 12th Symposium combustion. The Combustion Institute, Pettsburgh
Li J, Li YL, Guo H (2013) J Chem Phys 138:141102–141104
Purvis GD, Bartlett RJ (1982) J Chem Phys 76:1910–1918
Scuseria GE, Janssen CL, Schaefer HF (1988) J Chem Phys 89:7382–7387
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) Chem Phys Lett 157:479–483
Dunning TH (1989) J Chem Phys 90:1007–1023
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96:6796–6806
Woon DE, Dunning TH (1993) J Chem Phys 98:1358–1371
Wilson AK, Woon DE, Peterson KA, Dunning TH (1999) J Chem Phys 110:7667–7676
Peterson KA, Figgen D, Goll E, Stoll H, Dolg M (2003) J Chem Phys 119:11113–11123
CFOUR, a quantum chemical program package written by Stanton JF, Gauss J, Harding ME, Szalay PG, with contributions from Auer AA, Bartlett RJ, Benedikt U, Berger C, Bernholdt DE, Bomble YJ, Cheng L, Christiansen O, Heckert M, Heun O, Huber C, Jagau T-C, Jonsson D, Jusélius J, Klein K, Lauderdale WJ, Matthews DA, Metzroth T, O’Neill DP, Price DR, Prochnow E, Ruud K, Schiffmann F, Schwalbach W, Stopkowicz S, Tajti A, Vázquez J, Wang F, Watts JD, with the integral packages MOLECULE (Almlöf J, Taylor PR), PROPS (Taylor PR), ABACUS (Helgaker T, Jensen HJ Aa, Jorgensen P, Olsen J), and ECP routines by Mitin AV, Wullen C van (2010)
Hoy AR, Bunker PR (1979) J Mol Spectrosc 74:1–8
Benedict WS, Gailar N, Plyler EK (1956) J Chem Phys 24:1139–1165
Rank DH, Rao BS, Wiggins TA (1965) J Mol Spectrosc 17:122–130
Huber KP, Herzberg G (1979) Constants of diatomic molecules. Van Nostrand Reinhold Company, New York
Barletta P, Shirin SV, Zobov NF, Polyansky OL, Tennyson J, Valeev EF, Császár AG (2006) J Chem Phys 125:204307
Lide DR (2014) Structure of free molecules in the gas phase, in Section 9, CRC Handbook of Chemistry and Physics, 94th Edition. In: Haynes WM, (ed), CRC Press/Taylor and Francis Group, Boca Raton, FL
Li G, Zhou L, Li Q-S, Xie Y, Schaefer HF (2012) Phys Chem Chem Phys 14:10891–10895
Guo Y, Zhang M, Xie Y, Schaefer HF (2013) J Chem Phys 139:041101–041103
Schaefer HF (1985) J Chem Phys 89:5336–5343
Werner HJ, Kallay M, Gauss J (2008) J Chem Phys 128:034305–034308
Metz B, Stoll H, Dolg M (2000) J Chem Phys 113:2563–2569
Czakó G (2013) J Chem Phys 138:134301–134305
Moore CE (1971) Atomic energy levels, Volume II, page 159, NSRDS-NBS 35, Washington, DC
Ruscic B, Wagner AF, Harding LB, Asher RL, Feller D, Dixon DA, Peterson KA, Song Y, Qian X, Ng C-Y, Liu J, Chen W, Schwenke DW (2002) J Phys Chem A 106:2727–2747
Ruscic B (2014) Active thermochemical tables, version 1.112, Argonne national laboratory, Argonne, IL. http://atct.anl.gov
Linstrom PJ, Mallard WG (eds) (2014) NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD
Shenyavskaya EA, Yungman VS (2004) J Phys Chem Ref Data 33:923–957
Lide DR (2014) “Dipole Moments” in section 9. In: Haynes WM (ed) CRC handbook of chemistry and physics, 94th edn. CRC Press/Taylor and Francis Group, Boca Raton, FL
de Oliveira-Filho AGS, Ornellas FR, Bowman JM (2014) J Phys Chem Lett 5:706–712
Acknowledgments
We thank Dr. Gabor Czakó for very helpful discussions. Correspondence with Professors Antonio de Oliveira-Filho and Joel Bowman are sincerely appreciated. This research was supported by Tianjin Natural Science Foundation (11JCYBJC14500), the National Natural Science Foundation of China (Grant No. 10904111), and China Postdoctoral Science Foundation (20100470792), as well as by the U.S. Department of Energy, Office of Basic Energy Sciences, Chemical Sciences Division, Fundamental Interactions Branch.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor Isaiah Shavitt and published as part of the special collection of articles celebrating his many contributions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Hao, Y., Guo, Y. et al. Anchoring the potential energy surface for the Br + H2O → HBr + OH reaction. Theor Chem Acc 133, 1513 (2014). https://doi.org/10.1007/s00214-014-1513-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-014-1513-6