Abstract
Rationale
One of the key targets of psychopharmacology research is to determine the potential sites of action of antidepressants in order to characterise their underlying mechanism of action.
Objective
Using blood oxygenation level-dependent (BOLD) pharmacological magnetic resonance imaging (phMRI), the neuroanatomical target-sites of reboxetine (a selective noradrenaline reuptake inhibitor) and bupropion (an antidepressant with stimulatory effects on dopamine and potentially on noradrenaline) were mapped.
Methods
Separate groups of rats were challenged acutely or chronically (daily injections for 14 days) with saline or psychoactive compounds and scanned. Subsequent statistical parametric mapping of the main effects of the drug was performed by identifying changes in the BOLD signal.
Results
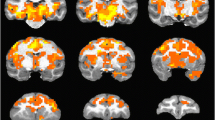
Acute reboxetine challenge at a low dose (10 mg/kg i.p.) produced positive BOLD responses specifically in the hypothalamus, whereas a larger dose (30 mg/kg i.p.) produced activations in the hypothalamus, anterior hippocampus and prefrontal cortex. Chronic reboxetine (30 mg/kg i.p.) treatment induced increased BOLD responses in the posterior hippocampus and prefrontal cortex, while no significant contrast changes were observed in the hypothalamus and a significant decrease was apparent in the amygdala. In contrast, acute bupropion (15 and 30 mg/kg i.p.) challenge in both doses produced no significant contrast changes in the regions of interest. However, chronic bupropion treatment (30 mg/kg i.p.) produced robust increases in BOLD responses in the hippocampus, amygdala and prefrontal cortex.
Conclusion
In summary, this study demonstrates that reboxetine and bupropion evoke a significant increase in BOLD functional activity in specific regions of the brain, including the hypothalamus, hippocampus, prefrontal cortex and amygdala. Furthermore, the study illustrates the potential value of pharmacological MRI in rodents to delineate pharmacologically induced changes in regional brain function.


Similar content being viewed by others
References
Beck C (1995) Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci 20:25–32
Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J (2010) Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci 30(12):4285–4294
Borsook D, Becerra L, Hargreaves R (2006) A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov 5:411–424
Bruhl AB, Kaffenberger T, Herwig U (2010) Serotonergic and noradrenergic modulation of emotion processing by single dose antidepressants. Neuropsychopharmacology 35:521–533
Brunello N, Blier P, Judd LL, Mendlewicz J, Nelson CJ, Souery D et al (2003) Noradrenaline in mood and anxiety disorders: basic and clinical studies. Int Clin Psychopharmacol 18:191–202
Chan CK, Durieux ME (1997) Differential inhibition of lysophosphatidate signaling by volatile anesthetics. Anesthesiology 86:660–669
Chuluunkhuu G, Nakahara N, Yanagisawa S, Kamae I (2008) The efficacy of reboxetine as an antidepressant, a meta-analysis of both continuous (mean HAM-D score) and dichotomous (response rate) outcomes. Kobe J Med Sci 54(2):147–158
Davidson RJ, Irwin W, Anderle MJ, Kalin NH (2003) The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160:64–75
Debiec J, LeDoux JE (2006) Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann NY Acad Sci 1071:521–524
Disbrow E, Baron R, Baron Y (1999) Examination of the role of the cerebral cortex in the perception of pain using functional magnetic resonance imaging. Curr Rev Pain 3:281–290
Dong J, Blier P (2001) Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacol Berl 155:52–57
Drevets WC (2000) Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 126:413–431
El Mansari M, Ghanbari R, Janssen S, Blier P (2008) Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacology 55:1191–1198
Ferris RM, White HL, Cooper BR, Maxwell RA, Tang FLM, Beaman OJ et al (1981) Some neurochemical properties of a new antidepressant, bupropion hydrochloride. Drug Dev Res 1:21–35
Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET (2004) Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. Sep;61(9):877–889.
Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM (1994) Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med 31:283–291
Harmer CJ (2010) Antidepressant drug action: a neuropsychological perspective. Depress Anxiety 27:231–233
Hlustik P, Noll DC, Small SL (1998) Suppression of vascular artifacts in functional magnetic resonance images using MR angiograms. Neuroimage 7:224–231
Invernizzi RW, Garattini S (2004) Role of presynaptic alpha2-adrenoceptors in antidepressant action: recent findings from microdialysis studies. Prog Neuropsychopharmacol Biol Psychiatry 28:819–827
Invernizzi RW, Parini S, Sacchetti G, Fracasso C, Caccia S, Annoni K et al (2001) Chronic treatment with reboxetine by osmotic pumps facilitates its effect on extracellular noradrenaline and may desensitize alpha(2)-adrenoceptors in the prefrontal cortex. Br J Pharmacol 132:183–188
Kastrup A, Baudewig J, Schnaudigel S, Huonker R, Becker L, Sohns JM, Dechent P, Klingner C, Witte OW (2008) Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage 41(4):1364–1371
Katzman MA, Tsirgielis D (2011) Review: reboxetine is ineffective and potentially harmful for acute treatment of major depression. Evid Based Ment Health (in press)
Lauritzen M (2008) On the neural basis of fMRI signals. Clin Neurophysiol 119:729–730
Leslie RA, James MF (2000) Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci 21:314–318
Lowe AS, Williams SC, Symms MR, Stolerman IP, Shoaib M (2002) Functional magnetic resonance neuroimaging of drug dependence: naloxone-precipitated morphine withdrawal. Neuroimage 17:902–910
Lowe AS, Beech JS, Williams SCR (2007) Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage 35:719–728
Lowe AS, Barker GJ, Beech JS, Ireland MD, Williams SC (2008) A method for removing global effects in small-animal functional MRI. NMR Biomed 21:53–58
Mateo Y, Fernandez-Pastor B, Meana JJ (2001) Acute and chronic effects of desipramine and clorgyline on alpha(2)-adrenoceptors regulating noradrenergic transmission in the rat brain: a dual-probe microdialysis study. Br J Pharmacol 133:1362–1370
McCabe C, Mishor Z, Cowen PJ, Harmer CJ (2010) Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67:439–445
Miskowiak K, Papadatou-Pastou M, Cowen PJ, Goodwin GM, Norbury R, Harmer CJ (2007) Single dose antidepressant administration modulates the neural processing of self-referent personality trait words. Neuroimage 37:904–911
Miyata S, Hamamura T, Lee Y, Miki M, Habara T, Oka T et al (2005) Contrasting Fos expression induced by acute reboxetine and fluoxetine in the rat forebrain: neuroanatomical substrates for the antidepressant effect. Psychopharmacol Berl 177:289–295
Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ (2007) Short-term antidepressant treatment and facial processing. Functional magnetic resonance imaging study. Br J Psychiatry 190:531–532.
Norbury R, Mackay CE, Cowen PJ, Goodwin GM, Harmer CJ (2008) The effects of reboxetine on emotional processing in healthy volunteers: an fMRI study. Mol Psychiatry 13:1011–1020
Onur OA, Walter H, Schlaepfer TE, Rehme AK, Schmidt C, Keysers C et al (2009) Noradrenergic enhancement of amygdala responses to fear. Soc Cogn Affect Neurosci 4:119–126
Page ME, Lucki I (2002) Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology 27:237–247
Papakostas GI, Nelson JC, Kasper S, Möller HJ (2008) A meta-analysis of clinical trials comparing reboxetine, a norepinephrine reuptake inhibitor, with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. Eur Neuropsychopharmacol 18(2):122–127
Peeters RR, Van der Linden A (2002) A data post-processing protocol for dynamic MRI data to discriminate brain activity from global physiological effects. Magn Reson Imaging. 20(6):503–510.
Preece M, Mukherjee B, Huang CL, Hall LD, Leslie RA, James MF (2001) Detection of pharmacologically mediated changes in cerebral activity by functional magnetic resonance imaging: the effects of sulpiride in the brain of the anaesthetised rat. Brain Res 916:107–114
Ressler KJ, Nemeroff CB (2001) Role of norepinephrine in the pathophysiology of neuropsychiatric disorders. CNS Spectr 6(663–6):670
Robertson B, Wang L, Diaz MT, Aiello M, Gersing K, Beyer J et al (2007) Effect of bupropion extended release on negative emotion processing in major depressive disorder: a pilot functional magnetic resonance imaging study. J Clin Psychiatry 68:261–267
Sacchetti G, Bernini M, Bianchetti A, Parini S, Invernizzi RW, Samanin R (1999) Studies on the acute and chronic effects of reboxetine on extracellular noradrenaline and other monoamines in the rat brain. Br J Pharmacol 128:1332–1338
Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H (2008) Negative BOLD with large increases in neuronal activity. Cereb Cortex. Aug;18(8):1814–1827. Epub 2007 Dec 5.
Sekar S, Verhoye M, Van Audekerke J, Vanhoutte G, Lowe AS, Blamire AM, Steckler T, Van der Linden A, Shoaib M (2011) Neuroadaptive responses to citalopram in rats using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 213:521–531
Sheline YI (2003) Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 54:338–352
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001) Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50:651–658
Shoaib M, Lowe AS, Williams SC (2004) Imaging localised dynamic changes in the nucleus accumbens following nicotine withdrawal in rats. Neuroimage 22:847–854
Steward CA, Prior MJW, Chapman V, Morris PG, Marsden CA (2004) Mapping functional changes in rat brain in response to altered serotonergic function using BOLD fMRI. Proc Intl Soc Mag Reson Med 11 (2004): 1169.
Sullivan GM, Coplan JD, Kent JM, Gorman JM (1999) The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry 46:1205–1218
Szabo ST, Blier P (2001) Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci 13:2077–2087
Szabo ST, de MC, Blier P (2000) Progressive attenuation of the firing activity of locus coeruleus noradrenergic neurons by sustained administration of selective serotonin reuptake inhibitors. Int J Neuropsychopharmacol 3:1–11
Tanaka M, Yoshida M, Emoto H, Ishii H (2000) Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 405:397–406
Wagner G, Koch K, Schachtzabel C, Sobanski T, Reichenbach JR, Sauer H et al (2010) Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. J Psychiatry Neurosci 35:247–257
Wise R, Tracey I (2006) The role of fMRI in drug discovery. J Magn Reson Imaging 23:862–876
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sekar, S., Van Audekerke, J., Vanhoutte, G. et al. Neuroanatomical targets of reboxetine and bupropion as revealed by pharmacological magnetic resonance imaging. Psychopharmacology 217, 549–557 (2011). https://doi.org/10.1007/s00213-011-2311-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2311-7