Abstract
Rationale
There is significant interest in the NMDA receptor antagonist ketamine due to its efficacy in treating depressive disorders and its induction of psychotic-like symptoms that make it a useful tool for modeling psychosis.
Objective
The present study extends the successful development of an apparatus and methodology to conduct pharmacological MRI studies in awake rhesus monkeys in order to evaluate the CNS effects of ketamine.
Methods
Functional MRI scans were conducted in four awake adult female rhesus monkeys during sub-anesthetic intravenous (i.v.) infusions of ketamine (0.345 mg/kg bolus followed by 0.256 mg/kg/h constant infusion) with and without risperidone pretreatment (0.06 mg/kg). Statistical parametric maps of ketamine-induced blood oxygenation level–dependent (BOLD) activation were obtained with appropriate general linear regression models (GLMs) incorporating motion and hemodynamics of ketamine infusion.
Results
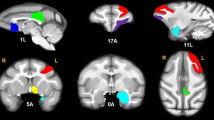
Ketamine infusion induced and sustained robust BOLD activation in a number of cortical and subcortical regions, including the thalamus, cingulate gyrus, and supplementary motor area. Pretreatment with the antipsychotic drug risperidone markedly blunted ketamine-induced activation in many brain areas.
Conclusions
The results are remarkably similar to human imaging studies showing ketamine-induced BOLD activation in many of the same brain areas, and pretreatment with risperidone or another antipsychotic blunting the ketamine response to a similar extent. The strong concordance of the functional imaging data in humans with these results from nonhuman primates highlights the translational value of the model and provides an excellent avenue for future research examining the CNS effects of ketamine. This model may also be a useful tool for evaluating the efficacy of novel antipsychotic drugs.




Similar content being viewed by others
References
Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–47
Abel KM, Drake R, Goldstein JM (2010) Sex differences in schizophrenia. Int Rev Psychiatry 22:417–28
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–5
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–4
Bolstad I, Andreassen OA, Groote I, Server A, Sjaastad I, Kapur S, Jensen J (2015) Effects of haloperidol and aripiprazole on the human mesolimbic motivational system: A pharmacological fMRI study. Eur Neuro Psychopharmacol
Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC, Sharma T, McGuire PK (1999) Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 7:38–48
Casey DE, Bruhwyler J, Delarge J, Geczy J, Liegeois JF (2001) The behavioral effects of acute and chronic JL 13, a putative antipsychotic, in Cebus non-human primates. Psychopharmacology (Berlin) 157:228–35
Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, Gonzalez-Rothi L, Briggs RW (2004) Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci 16:167–77
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–73
Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T (2001) Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Brain Res Rev 37:153–61
De Simoni S, Schwarz AJ, O'Daly OG, Marquand AF, Brittain C, Gonzales C, Stephenson S, Williams SC, Mehta MA (2013) Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage 64:75–90
Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65:154–64
Doan L, Manders T, Wang J (2015) Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast 2015: 504691
Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O'Daly OG, Williams SC, Mehta MA (2013) Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J Pharmacol Exp Ther 345:151–60
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–47
Frohlich J, Van Horn JD (2014) Reviewing the ketamine model for schizophrenia. J Psychopharmacol 28:287–302
Haber SN (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26:317–30
Howell LL, Fantegrossi WE (2009) Intravenous Drug Self-Administration in Nonhuman Primates. In: Buccafusco JJ (ed) Methods of Behavior Analysis in Neuroscience (Frontiers in Neuroscience), Boca Raton (FL)
Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS (2008) Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex 18:1374–83
Joules R, Doyle OM, Schwarz AJ, O'Daly OG, Brammer M, Williams SC, Mehta MA (2015) Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology (Berlin)
Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH (2007) From the cover: antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A 104:3456–9
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73:1133–41
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–34
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–64
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–52
Mayberg HS (2003) Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–60
McGregor KM, Sudhyadhom A, Nocera J, Seff A, Crosson B, Butler AJ (2015) Reliability of negative BOLD in ipsilateral sensorimotor areas during unimanual task activity. Brain Imaging Behav 9:245–54
Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–55
Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104
Moffett K, Crosson B, Spence JS, Case K, Levy I, Gopinath K, Shah P, Goyal A, Fang Y, Briggs RW, Hart J Jr, Moore A, Haley RW (2015) Word-finding impairment in veterans of the 1991 Persian Gulf War. Brain Cogn 98:65–73
Morgan CJ, Perry EB, Cho HS, Krystal JH, D'Souza DC (2006) Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology (Berlin) 187:405–14
Murnane KS, Howell LL (2010) Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods 191:11–20
Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL (2015) Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology (Berlin) 232:745–54
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007
Paxinos G, Huang XF, Toga AW (2000) The rhesus monkey brain in stereotaxic coordinates. Academic Press, San Diego
Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML (2014) Why primate models matter. Am J Primatol 76:801–27
Preuss TM (1995) Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci 7:1–24
Rowland LM (2005) Subanesthetic ketamine: how it alters physiology and behavior in humans. Aviat Space Environ Med 76:C52–8
Schmechtig A, Lees J, Perkins A, Altavilla A, Craig KJ, Dawson GR, William Deakin JF, Dourish CT, Evans LH, Koychev I, Weaver K, Smallman R, Walters J, Wilkinson LS, Morris R, Williams SC, Ettinger U (2013) The effects of ketamine and risperidone on eye movement control in healthy volunteers. Trans Psychiatry 3:e334
Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M (2009) Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain 147:107–15
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–19
Stone JM (2011) Glutamatergic antipsychotic drugs: a new dawn in the treatment of schizophrenia? Ther Adv Psychopharmacol 1:5–18
Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A (2003) Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry 60:133–41
Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X (2015) An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294
van den Buuse M, Mingon RL, Gogos A (2015) Chronic estrogen and progesterone treatment inhibits ketamine-induced disruption of prepulse inhibition in rats. Neurosci Lett 607:72–6
Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL (2000) Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34:35–43
Weiser M, Heresco-Levy U, Davidson M, Javitt DC, Werbeloff N, Gershon AA, Abramovich Y, Amital D, Doron A, Konas S, Levkovitz Y, Liba D, Teitelbaum A, Mashiach M, Zimmerman Y (2012) A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry 73:e728–34
Young E, Korszun A (2010) Sex, trauma, stress hormones and depression. Mol Psychiatry 15:23–8
Acknowledgments
The authors declare no competing financial interests. This research was supported by P51OD11132 (Yerkes National Primate Research Center), Sunovion Pharmaceutical, Ltd. (LLH) and DA031246 (LLH). Special thanks to the Yerkes Imaging Center, technicians Marisa Olsen and Juliet Brown, and to Christopher Muly, MD, PhD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eric Maltbie and Kaundinya Gopinath contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 149 kb)
Online Resource 2
(PDF 121 kb)
Rights and permissions
About this article
Cite this article
Maltbie, E., Gopinath, K., Urushino, N. et al. Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233, 961–972 (2016). https://doi.org/10.1007/s00213-015-4175-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4175-8