Abstract.
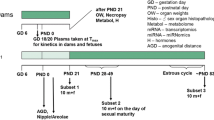
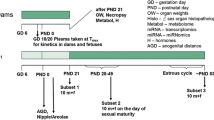
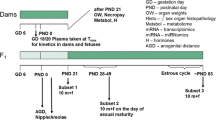
In association with the international validation project to establish an OECD Enhanced Test Guideline 407, we performed a 28-day repeated-dose toxicity study of genistein, which is known as a phytoestrogen. Attention was paid to the sensitivity of certain additional parameters, such as histopathology observations and organ weights of endocrine related organs, sperm characteristics, serum hormone levels and estrous cycle, for detecting endocrine-related effects of endocrine-disrupting chemicals based on the existing TG 407. Seven-week-old Crj:CD(SD)IGS rats were assigned to one of four groups, each consisting of ten males and ten females, and genistein was administered once daily by gavage at doses of 0 (control), 120, 400 or 1000 mg/kg body weight per day. Male rats were killed on the day after the 28th administration. Female rats were killed on the day of the diestrus stage during the 4 days after the 28th administration. Endocrine-disrupting effects of genistein were detected in females by histopathology. The changes included vacuolation and mucinification of the vaginal epithelium in the 400 and 1000 mg/kg groups; however, the incidences of the lesion were very low. Although increased serum prolactin levels were recorded in the males of the 1000 mg/kg group, we could not determine whether this was indeed induced by genistein. General toxicological effects of genistein were detected in blood chemistry, such as increased triglycerides and total protein and a decreased albumin/globulin ratio, as well as increased liver weight and glycogen deposition in the periportal hepatocytes. Based on these results, the no-observed-adverse-effect level (NOAEL) in the present study was estimated to be 120 mg/kg per day. In particular, endocrine-related effects were most sensitively detected by histopathology examination of sexual organs. However, the findings indicate that chemicals with weak endocrine-disrupting potential like genistein must be evaluated taking into consideration the results of other test systems.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Okazaki, K., Okazaki, S., Nakamura, H. et al. A repeated 28-day oral dose toxicity study of genistein in rats, based on the 'Enhanced OECD Test Guideline 407' for screening endocrine-disrupting chemicals. Arch Toxicol 76, 553–559 (2002). https://doi.org/10.1007/s00204-002-0376-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00204-002-0376-0