Abstract
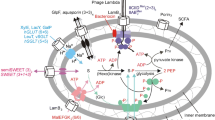
In Escherichia coli K-12, transcription of the ferric citrate transport genes fecABCDE is initiated by binding of diferric dicitrate to the outer membrane protein FecA which elicits a signaling cascade from the cell surface to the cytoplasm. The FecI sigma factor is only active in the presence of FecR, which transfers the signal across the cytoplasmic membrane. In other bacteria, fecIRA homologues control iron transport gene transcription by siderophores other than citrate. However, in most cases, the FecI homologues are active in the absence of the FecR homologues, which might function as anti-sigma factors. Since not all E. coli strains contain a fec system, we determined the occurrence of fec genes in selected Enterobacteriaceae and the dependence of FecI activity on FecR. Incomplete FecIRA systems were chromosomally encoded in Enterobacter aerogenes strains and plasmid-encoded in K. pneumoniae. E. coli B, Photorhabdus luminescens and one of three Klebsiella pneumoniae strains had a functional FecIRA regulatory system as in E. coli K-12. The cytoplasmic N-terminal FecR fragments caused constitutive FecI activity in the absence of ferric citrate. The PCR-generated mutant FecI(D40G) was inactive and FecI(S15P) was partially active. FecR of E. coli K-12 activated FecI of all tested strains except FecI encoded on the virulence plasmid pLVPK of K. pneumoniae, which differed from E. coli K-12 FecI by having mutations in region 4, which is important for interaction with FecR. The C-terminally truncated FecR homologue of pLVPK was inactive. pLVPK-encoded FecA contains a 38-residue sequence in front of the signal sequence that did not prevent processing and proper integration of FecA into the outer membrane of E. coli and lacks the signaling sequence required for transcription initiation of the fec transport genes, making it induction-incompetent but transport-competent. The evidence indicates that fecIRABCDE genes are acquired by horizontal DNA transfer and can undergo debilitating mutations.



Similar content being viewed by others
References
Angerer A, Enz S, Ochs M, Braun V (1995) Transcriptional regulation of ferric citrate transport in Escherichia coli K-12 FecI belongs to a new subfamily of σ70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol 18:163–174
Angerer A, Braun V (1998) Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch Microbiol 169:483–490
Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, ShaoY (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474
Braun V (1995) Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev 16:295–307
Braun V, Mahren S, Ogierman M (2003) Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr Opin Microbiol 6:173–180
Brito B, Aldon D, Barberis P, Boucher C, Genin S (2002) A signal transfer system through three compartments transduces the plant cell contact-dependent signal controlling Ralstonia solanacearum hrp genes. Mol Plant Microbe Interact 15:109–119
Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL (2004) Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337:189–198
Ciche TA, Blackburn M, Carney JR, Ensign JC (2003) Photobactin, a catechol siderophore produced by Photorhabdus luminescens, an entomopathogen mutually associated with Heterorhabditis bacteriophora NC1 nematodes. Appl Environ Microbiol 69:4706–4713
Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux–Eude C, Chandler M, Charles JF, Dassa E, Derose R, Derzelle S, Freyssinet G, Gaudriault S, Medigue C, Lanois A, Powell K, Siguier P, Vincent R, Wingate V, Zouine M, Glaser P, Boemare N, Danchin A, Kunst F (2003) The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol 21:1307–1313
Enz S, Braun V, Crosa J (1995) Transcription of the region encoding the ferric dicitrate transport system in Escherichia coli: similarity between promoters for fecA and for extracytoplasmic function sigma factors. Gene 163:13–18
Enz S, Mahren S, Stroeher UH, Braun V (2000) Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR and FecI regulatory proteins. J Bacteriol 182:637–646
Enz S, Brand H, Orellana C, Mahren S, Braun V (2003) Sites of interaction between the FecA and FecR signal transduction proteins of ferric citrate transport in Escherichia coli K-12. J Bacteriol 185:3745–3752
Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J (2002) Structural basis of gating by the outer membrane transporter FecA. Science 295:1715–1719
Frost GE, Rosenberg H (1973) The inducible citrate–dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta 330:90–101
Giacomini A, Corich B, Ollero FJ, Squartini A, Nuti MP (1992) Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol Lett 100:87–90
Härle C, Insook K, Angerer A, Braun V (1995) Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J 14:1430–1438
Helmann JD (2002) The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol 46:47–110
Hochhut B, Dobrindt U, Hacker J (2005) Pathogenicity islands and their role in bacterial virulence and survival. In: Russel W, Herwald H, (eds) Concepts in bacterial virulence. Karger, Basel, pp 234–254
Hussein S, Hantke K, Braun V (1981) Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem 117:431–437
Kim I, Stiefel A, Plantör S, Angerer A, Braun V (1997) Transcription induction of the ferric citrate transport genes via the N terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol Microbiol 23:333–344
Kirby AE, King ND, Connell TD (2004) RhuR, an extracytoplasmic function sigma factor activator, is essential for heme-dependent expression of the outer membrane heme and hemoprotein receptor of Bordetella avium. Infect Immun 72:896–907
Koster M, van Klompenburg W, Bitter W, Leong J, Weisbeek P (1994) Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J 13:2805–2813
Luck SN, Turner SA, Rajakumar K, Sakellaris H, Adler B (2001) Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect Immun 69:6012–6021
Mahren S, Enz S, Braun V (2002) Functional interaction of region 4 of the extracytoplasmic function sigma factor FecI with the cytoplasmic portion of the FecR transmembrane protein of the Escherichia coli ferric citrate transport system. J Bacteriol 184:3704–3711
Mahren S, Braun V (2003) The FecI extracytoplasmic-function sigma factor of Escherichia coli interacts with the beta’ subunit of RNA polymerase. J Bacteriol 185:1796–1802
Miller J H (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Murakami KS, Masuda S, Darst SA (2002) Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science 296:1280–1284
Ochs M, Veitinger S, Kim I, Welz D, Angerer A, Braun V (1995) Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol Microbiol 15:119–132
Ochs M, Angerer A, Enz S, Braun V (1996) Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fec transport gene transcription. Mol Gen Genet 250:455–465
Ogierman M, Braun V (2003) Interactions between the outer membrane ferric citrate transporter FecA and TonB: studies of the FecA TonB box. J Bacteriol 185:1870–1885
Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MT, Sebaihia M, Baker S, Basham D, Brooks K, Chillingworth T, Connerton P, Cronin A, Davis P, Davies RM, Dowd L, White N, Farrar J, Feltwell T, Hamlin N, Haque A, Hien TT, Holroyd S, Jagels K, Krogh A, Larsen TS, Leather S, Moule S, O’Gaora P, Parry C, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG (2001) Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852
Perna NT, Plunkett G III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta E, Potamousis K, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR (2001) Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529–533
Pradel E, Locht C (2001) Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J Bacteriol 183:2910–2907
Pressler U, Staudenmaier H, Zimmermann L, Braun V (1988) Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol 170:2716–2724
Redly GA, Poole K (2003) Pyoverdine-mediated regulation of FpvA synthesis in Pseudomonas aeruginosa: involvement of a probable extracytoplasmic-function sigma factor, FpvI. J Bacteriol 185:1261–1265
Rossi MS, Paquelin A, Ghigo JM, Wandersman C (2003) Haemophore-mediated signal transduction across the bacterial cell envelope in Serratia marcescens: the inducer and the transported substrate are different molecules. Mol Microbiol 48:1467–1480
Silhavy TS, Bermann ml, Enquist LW (1994) Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Tabor S, Richardson CC (1985) A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82:1074–1078
Takeshita S, Sato M, Toba M, Masahashi M, T Hashimoto-Gotoh (1987) High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74
Stiefel A, Mahren S, Ochs M, Schindler PT, Enz S, Braun V (2001) Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane regulatory protein. J Bacteriol 183:162–170
Van hove B, Staudenmaier H, Braun V (1990) Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol 172:6749–6758
Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 417:712–719
Welz D, Braun V (1998) Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J Bacteriol 180:2387–2394
Yue WW, Grizot S, Buchanan SK (2003) Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J Mol Biol 332:353–368
Acknowledgements
We thank K. Hantke for fruitful discussions, C. Herrmann for performing the transport experiments, and K. A. Brune for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahren, S., Schnell, H. & Braun, V. Occurrence and regulation of the ferric citrate transport system in Escherichia coli B, Klebsiella pneumoniae, Enterobacter aerogenes, and Photorhabdus luminescens. Arch Microbiol 184, 175–186 (2005). https://doi.org/10.1007/s00203-005-0035-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-005-0035-y