Abstract
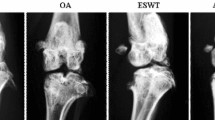
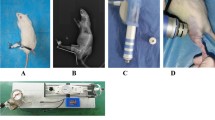
This study investigated the molecular changes of DKK-1, MMP13, Wnt-5a and \(\upbeta \)-catenin after extracorporeal shockwave therapy (ESWT) in anterior cruciate ligament transected (ACLT) osteoarthritic (OA) knee in rats. 27 male Spraque-Dawley rats were divided into three groups. Group I was the control one and received sham knee arthrotomy but no ACLT or ESWT. Group II underwent ACLT, but no ESWT. Group III underwent ACLT and received ESWT. The animals were killed at 12 weeks, and the harvested knee specimens were subjected to histopathological examination and immunohistochemical analysis. Radiographs of the knees were obtained at 0 and 12 weeks. At 12 weeks, radiographs of group II showed more arthritic changes with formation of osteochondral fragments, whereas very subtle arthritis was noted in groups I and III. In histopathological examination, group II showed a significant increase of Mankin score and a decrease of subchondral bone as compared to groups I and III. Group III showed a significant decrease of Mankin score and an increase of subchondral bone, with the data comparable to group I. In immunohistochemical analysis, group II showed significant increases of DKK-1 and MMP13 and decreases of Wnt-5a and \(\upbeta \)-catenin in articular cartilage and subchondral bone as compared to groups I and III. Group III showed significant decreases of DKK-1 and MMP13 and increases of Wnt-5a and \(\upbeta \)-catenin, with the data comparable to group I. In conclusion, the application of ESWT causes molecular changes that are consistent with the improvement in subchondral bone remodeling and chondroprotective effect in ACLT OA knees in rats.



Similar content being viewed by others
References
Oettmeier, R., Abendroth, K.: Osteoarthritis and bone:osteologic types of osteoarthritis of the hip. Skeletal. Radiol. 18, 165–174 (1989)
Lane, N.E., Nevitt, M.C.: Osteoarthritis, bone mass, and fractures: how are they related? Arthritis. Rheum. 46, 1–4 (2002)
Mankin, H.J., Dorfman, H., Lippiello, L., Zarins, A.: Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips: II. Correlation of morphology with biochemical and metabolic data. J. Bone. Joint. Surg. Am. 53, 523–537 (1971)
Hayami, T., Funaki, H., Yaoeda, K., Mitui, K., Yamagiwa, H., Tokunaga, K., et al.: Expression of the cartilage-derived anti-angiogenic factor chondromudulin-I decreases in the early stage of experimental osteoarthritis. J. Rheumatol. 30, 2207–2217 (2003)
Little, C., Smith, S., Ghosh, P., Bellenger, C.: Histopathological and immunohistochemical evaluation of joint changes in a model of osteoarthritis induced by lateral meniscectomy in sheep. J. Rheumatol. 24, 2199–2209 (1997)
Radin, E.L., Rose, R.M.: Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. 213, 34–40 (1986)
Burr, D.M., Schaffler, M.B.: The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Micros. Res. Tech. 37, 343–357 (1997)
Burr, D.B.: The importance of subchondral bone in osteoarthrosis. Curr. Opin. Rheumatol. 10, 256–262 (1998)
Dedrick, D.K., Goulet, R., Huston, L., Goldstein, S.A., Bole, G.G.: Early bone changes in experimental osteoarthritis using microscopic computed tomography. J. Rheumatol. Suppl. 27, 44–45 (1991)
Goker, B., Sumner, D.R., Hurwitz, D.E., Block, J.A.: Bone mineral density varies as a function of the rate of joint space narrowing in the hip. J. Rheumatol. 27, 735–738 (2000)
Ratcliffe, A., Seibel, M.J.: Biochemical markers of osteoarthritis. Curr. Opin. Rheumatol. 277, 21352–21360 (1990)
Harada, S.I., Rodan, G.A.: Control of osteoblast function and regulation of bone mass. Nature 423, 35–41 (2003)
Wang, C.J., Weng, L.H., Ko, J.Y., Sun, Y.C., Yang, Y.J., Wang, F.S.: Extracorporeal Shockwave Therapy Shows Chondroprotective Effects in Osteoarthritic Rat Knee. Arch. Orthop. Trauma. Surg. 131, 1153–1158 (2011)
Takahashi, K., Yamazaki, M., Saisu, T., et al.: Gene expression for extracellular matrix proteins in shockwave-induced osteogenesis in rats. Calcif. Tissue. Int. 74, 187–193 (2004)
Aguilera, O., Munoz, A., Esteller, M., Fraga, M.F.: Epigenetic alterations of the Wnt-beta catenin pathway in human disease. Endocr. Metab. Immune. Disord. Drug. Targets. 7, 13–21 (2007)
Hall, C.L., Keller, E.T.: The role of Wnt in bone metastases. Cancer. Metastasis. Rev. 25, 551–558 (2006)
Nishimura, R., Yoneda, T.: Role of Wnt in bone formation. Clin. Calcium. 16, 817–822 (2006)
Aslan, H., Ravid-Amir, O., Clancy, B.M., et al.: Advanced molecular profiling in vivo detects novel function of dickkopf-3 in the regulation of bone formation. J. Bone. Mineral. Res. 21, 1935–1945 (2006)
Niehrs, C.: Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469–7481 (2006)
MacDonald, B.T., Joiner, D.M., Oyserman, S.M., Sharma, P., Goldstein, S.A., He, X., Hauschka, P.V.: Bone mass is inversely proportional to DKK-1 levels in mice. Bone 41, 331–339 (2007)
Li, J., Sarosi, I., Cattley, R.C., et al.: DKK-1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39, 754–766 (2006)
Morvan, F., Boulukos, K., Clement-Lacroix, P., et al.: Detection of a single allele of the DKK-1 gene leads to an increase in bone formation and bone mass. J. Bone. Mineral. Res. 21, 934–945 (2006)
Ohnaka, K., Taniguchi, H., Kawate, H., Nawata, H., Takayanagi, R.: Glucocorticoid enhances the expression of dickkopf-1 in human osteoblast: novel mechanism of glucocorticoid-induced osteoporosis. Biochem. Biophys. Res. Commun. 318, 259–264 (2004)
Pritzker, K.P., Gay, S., Jimenez, S.A., Ostergaard, K., Pelletier, J.P., Revell, P.A., Salter, D., van den Berg, W.B.: Osteoarthritis cartilage histopathology grading and staging. Osteoarthr. Cartil. 14, 13–29 (2006)
Bettica, P., Cline, G., Hart, D.J., Meyer, J., Spector, T.D.: Evidence for increased bone resorption in patients with progressive knee osteoarthritis: Longitudinal results from the Chingford study. Arthritis Rheum. 46, 3178–3184 (2002)
Botter, S.M., van Osch, G.J., Waarsing, J.H., et al.: Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology 43, 379–388 (2006)
Hayami, T., Pickarski, M., Zhuo, Y., Wesolowski, G.A., Rodan, G.A., Duong, L.T.: Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38, 234–243 (2006)
Henson, F.M.D., Vincent, T.A.: Alterations in the vimentin cytoskeleton in response to single impact load in an in vitro model of cartilage damage in the rat. BMC Musculoskelet. Disord. 9, 94 (2008)
Acknowledgments
Grants were received in total and partial in the support of this study. The source of grants is National Science Council (NSC 98-2314-B-182A-010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. H. R. Hosseini.
Appendix
Rights and permissions
About this article
Cite this article
Wang, CJ., Sun, YC., Wu, CT. et al. Molecular changes after shockwave therapy in osteoarthritic knee in rats. Shock Waves 26, 45–51 (2016). https://doi.org/10.1007/s00193-013-0480-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00193-013-0480-5