Abstract
Purpose
Endothelial cell activation and dysfunction are involved in the pathophysiology of ARDS. Circulating endothelial cells (CECs) may be a useful marker of endothelial dysfunction and damage but have been poorly studied in ARDS. We hypothesized that the CEC count may be elevated in patients with sepsis-related ARDS compared to those with sepsis without ARDS.
Methods
ARDS was defined according to the Berlin consensus definition. The study population included 17 patients with moderate or severe ARDS, 9 with mild ARDS, 13 with sepsis and no ARDS, 13 non-septic patients, and 12 healthy volunteers. Demographic, hemodynamic, and prognostic variables, including PaO2/FiO2 ratio, 28-day survival, blood lactate, APACHE II, and SOFA score, were recorded. CECs were counted in arterial blood samples using the reference CD146 antibody-based immunomagnetic isolation and UEA1-FITC staining method. Measurements were performed 12–24 h after diagnosis of ARDS and repeated daily for 3 days.
Results
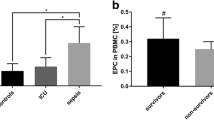
The median day-1 CEC count was significantly higher in patients with moderate or severe ARDS than in mild ARDS or septic-control patients [27.2 (18.3–49.4) vs. 17.4 (11–24.5) cells/ml (p < 0.034), and 18.4 (9.1–31) cells/ml (p < 0.035), respectively]. All septic patients (with or without ARDS) had higher day-1 CEC counts than the non-septic patients [19.6 (14.2–30.6) vs. 10.8 (5.7–13.2) cells/ml, p = 0.002].
Conclusion
The day-1 CEC count was significantly higher in ARDS patients than in other critically ill patients, and in moderate or severe ARDS patients compared to those with milder disease, making it a potentially useful marker of ARDS severity.


Similar content being viewed by others
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Zambon M, Vincent J-L (2008) Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133:1120–1127
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, PROSEVA Study Group (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368:2159–2168
Peek GJ, Elbourne D, Mugford M, Tiruvoipati R, Wilson A, Allen E, Clemens F, Firmin R, Hardy P, Hibbert C, Jones N, Killer H, Thalanany M, Truesdale A (2010) Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess Winch Engl 14:1–46
Orfanos SE, Mavrommati I, Korovesi I, Roussos C (2004) Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med 30:1702–1714
Wort SJ, Evans TW (1999) The role of the endothelium in modulating vascular control in sepsis and related conditions. Br Med Bull 55:30–48
Johnson ER, Matthay MA (2010) Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 23:243–252
Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA (2004) Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med 170:766–772
Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA, NHLBI ARDS Network (2008) Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 63:1083–1089
Eisner MD, Parsons P, Matthay MA, Ware L, Greene K, Acute Respiratory Distress Syndrome Network (2003) Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 58:983–988
Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M (2005) Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 172:854–860
Yoder MC (2009) Defining human endothelial progenitor cells. J Thromb Haemost 7(Suppl 1):49–52
Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, Sampol J, Solovey A, Dignat-George F (2005) Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost 93:228–235
Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F (2006) Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost JTH 4:671–677
Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, Baudouin SV (2001) Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med 163:195–200
Schlichting DE, Waxman AB, O’Brien LA, Wang T, Naum CC, Rubeiz GJ, Um SL, Williams M, Yan SC (2011) Circulating endothelial and endothelial progenitor cells in patients with severe sepsis. Microvasc Res 81:216–221
Moussa MD, Santonocito C, Fagnoul D, Donadello K, Pradier O, Gaussem P, De Backer D, Vincent JL (2012) Evaluation of endothelial damage in sepsis-related ARDS using circulating endothelial cell counts. Crit Care Med 12:105 (abst)
Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Smadja DM, Mauge L, Sanchez O, Silvestre JS, Guerin C, Godier A, Henno P, Gaussem P, Israël-Biet D (2010) Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J 36:1284–1293
Smadja DM, Gaussem P, Mauge L, Israël-Biet D, Dignat-George F, Peyrard S, Agnoletti G, Vouhé PR, Bonnet D, Lévy M (2009) Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation 119:374–381
Wen J, Chen J, Ji S-M, Cheng D, Liu ZH (2012) Evaluation of vascular lesions using circulating endothelial cells in renal transplant patients. Clin Transplant 26:E344–E350
Zimmerman GA, Albertine KH, Carveth HJ, Gill EA, Grissom CK, Hoidal JR, Imaizumi T, Maloney CG, McIntyre TM, Michael JR, Orme JF, Prescott SM, Topham MS (1999) Endothelial activation in ARDS. Chest 116:18S–24S
Meyrick B (1986) Pathology of the adult respiratory distress syndrome. Crit Care Clin 2:405–428
Walenta KLH, Link A, Friedrich EB, Böhm M (2009) Circulating microparticles in septic shock. Am J Respir Crit Care Med 180:100
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M (2005) Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 172:854–860
Gao X, Chen W, Liang Z, Chen L (2011) Autotransplantation of circulating endothelial progenitor cells protects against lipopolysaccharide-induced acute lung injury in rabbit. Int Immunopharmacol 11:1584–1590
Cribbs SK, Sutcliffe DJ, Taylor WR, Rojas M, Easley KA, Tang L, Brigham KL, Martin GS (2012) Circulating endothelial progenitor cells inversely associate with organ dysfunction in sepsis. Intensive Care Med 38:429–436
Rafat N, Hanusch C, Brinkkoetter PT, Schulte J, Brade J, Zijlstra JG, van der Woude FJ, van Ackern K, Yard BA, Beck GCh (2007) Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med 35:1677–1684
Hristov M, Erl W, Weber PC (2003) Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med 13:201–206
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95:952–958
Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109:1801–1809
Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS (2006) Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res 16:577–584
Kotb NA, Gaber R, Salah W, Elhendy A (2012) Relations among glycemic control, circulating endothelial cells, nitric oxide, and flow mediated dilation in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 120:460–465
Karthikeyan VJ, Blann AD, Baghdadi S, Lane DA, Gareth Beevers D, Lip GY (2011) Endothelial dysfunction in hypertension in pregnancy: associations between circulating endothelial cells, circulating progenitor cells and plasma von Willebrand factor. Clin Res Cardiol 100:531–537
Boos CJ, Blann AD, Lip GYH (2007) Assessment of endothelial damage/dysfunction: a focus on circulating endothelial cells. Methods Mol Med 139:211–224
Lee KW, Lip GYH, Tayebjee M, Foster W, Blann AD (2005) Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood 105:526–532
George F, Poncelet P, Laurent JC, Massot O, Arnoux D, Lequeux N, Ambrosi P, Chicheportiche C, Sampol J (1991) Cytofluorometric detection of human endothelial cells in whole blood using S-Endo 1 monoclonal antibody. J Immunol Methods 139:65–75
Acknowledgments
The authors thank Dr. Hassane Njimi for his help with the statistical analysis. We are also grateful to Sébastien Bertil and Florence Desvard for their technical assistance in CEC isolation.
Conflicts of interest
The authors have no conflicts of interest related to this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moussa, M.D., Santonocito, C., Fagnoul, D. et al. Evaluation of endothelial damage in sepsis-related ARDS using circulating endothelial cells. Intensive Care Med 41, 231–238 (2015). https://doi.org/10.1007/s00134-014-3589-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3589-9