Abstract
Angiopoietin-2 (Ang-2) is associated with vascular endothelial injury and permeability in the acute respiratory distress syndrome (ARDS) and sepsis. Elevated circulating Ang-2 levels may identify critically ill patients with distinct pathobiology amenable to targeted therapy. We hypothesized that plasma Ang-2 measured shortly after hospitalization among patients with sepsis would be associated with the development of ARDS and poor clinical outcomes. To test this hypothesis, we measured plasma Ang-2 in a cohort of 757 patients with sepsis, including 267 with ARDS, enrolled in the emergency department or early in their ICU course before the COVID-19 pandemic. Multivariable models were used to test the association of Ang-2 with the development of ARDS and 30-day morality. We found that early plasma Ang-2 in sepsis was associated with higher baseline severity of illness, the development of ARDS, and mortality risk. The association between Ang-2 and mortality was strongest among patients with ARDS and sepsis as compared to those with sepsis alone (OR 1.81 vs. 1.52 per log Ang-2 increase). These findings might inform models testing patient risk prediction and strengthen the evidence for Ang-2 as an appealing biomarker for patient selection for novel therapeutic agents to target vascular injury in sepsis and ARDS.
Similar content being viewed by others
Introduction
Biomarker-guided patient selection has the potential to match specific biologic therapies with the most relevant pathophysiology and therefore with the greatest potential for benefit in acute respiratory distress syndrome (ARDS). The angiopoietin (Ang)-Tie pathway is a key regulator of vascular endothelial permeability that contributes to non-cardiogenic pulmonary edema in critically ill patients [1]. In ARDS and sepsis, antagonism of Tie2 signaling by soluble Ang-2 inhibitors disrupts endothelial cell tight junctions, leading to increased vascular permeability and inflammation (reviewed in [2]). Previous small prospective cohort studies of critically ill patients suggest an association between plasma Ang-2 and development of ARDS [3] and poor clinical outcomes in patients with sepsis, including those with respiratory failure [4, 5]. We hypothesized that higher plasma Ang-2 measured shortly after hospitalization (usually within 24 h) would be associated with worse clinical outcomes in a large cohort of critically ill patients with sepsis. More specifically, we hypothesized that early plasma Ang-2 would be associated with a higher incidence of developing ARDS and increased mortality after adjusting for relevant clinical and demographic features.
Methods
We conducted a retrospective analysis of 758 prospectively enrolled septic patients admitted to the ICU from the emergency department in the EARLI (Early Assessment of Renal and Lung Injury study) cohort between October 2008 and August 2018 [3], 267 of whom developed ARDS according to the Berlin Definition within the first five days of ICU admission [6]. Sepsis was defined as meeting at least 2 SIRS criteria (> 38.0 °C or hypothermia < 36.0 °C, tachycardia > 90 beats/minute, tachypnea > 20 breaths/minute, leukocytosis > 12 × 109 cells/L or leukopenia < 4 × 109 cells/L) and confirmed positive microbiological culture or clinically diagnosed sepsis adjudicated by a research physician. The study was approved by the Institutional Review Board of the University of California, San Francisco. Procedures for informed consent have been previously described [3].
Plasma Ang-2 levels were measured using a Luminex™ multiplexed bead-based assay or ELISA (R&D™). Baseline (first available) Ang-2 values were used in all analyses, using loge-transformed values in analyses using Ang-2 as a continuous predictor variable. To assess the temporal relationship between elevated Ang-2 and ARDS development, we performed a subgroup analysis comparing Ang-2 levels between at-risk subjects who developed ARDS more than 24 h after plasma sampling (n = 78) to at-risk subjects who did not develop ARDS during our observation period (n = 408). This analysis excluded patients with ARDS identified within 24 h of ICU admission, for whom the timing of ARDS diagnosis was not clear, or for whom ARDS plasma was collected more than 24 h after enrollment.
Continuous variables are presented as median (IQR) and compared using Wilcoxon rank-sum tests. Categorical variables were compared using the Chi-square test. Adjusted logistic regression models (age and sex) were used to estimate the association between Ang-2 and 30-day mortality among all subjects. A separate analysis including an interaction term between log-transformed Ang-2 and ARDS was used to test whether ARDS mediated the association between Ang-2 and mortality. Adjustment for severity of illness scores was not included in these models as Ang-2 is on the same causal pathways as organ failures in our theoretical causal model. A two-sided P value less than 0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R.
Results
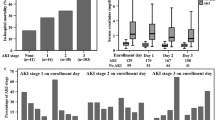
Demographic characteristics of all septic patients and of those with sepsis and ARDS are presented in Table 1. At day 30, 25% of patients with sepsis in the overall cohort had died as compared with 39% of patients with both sepsis and ARDS. Baseline plasma Ang-2 was significantly associated with 30-day mortality in adjusted logistic regression models. In models adjusting for age and sex, a one unit change in log plasma Ang-2 was associated with 1.52-fold increase in the odds of death (95% CI 1.28–1.80) in the full cohort (of septic patients (n = 757) and a 1.81-fold increase (95% CI 1.37–2.40) in the patients with both sepsis and ARDS (n = 267) (Table 2). In a separate logistic regression model of 30-day mortality including an interaction term between Ang-2 and ARDS, there was not a statistically significant interaction (p = 0.071).
Plasma samples were collected within 24 h of ICU admission for greater than 96% of patients, and within 48 h of for all patients for whom the timing of sample collection was recorded. Median (IQR) Ang-2 levels were higher among 78 sepsis patients in whom plasma was sampled more than 24 h prior to meeting Berlin ARDS diagnostic criteria compared to a group of 408 at-risk sepsis patients who did not develop ARDS during the first 5 days of hospitalization [7577 pg/ml (4216–16,699 pg/ml) vs. 6032 pg/ml (3009–11,821 pg/ml), p = 0.02].
Among all patients with sepsis, median Ang-2 levels were higher in patients requiring vasopressors on ICU Day 1 compared with patients who did not. Patients who required vasopressors on day 1 in ICU had Ang-2 median levels of 8426 (3763–16,000) compared to 5000 (2587–9334) for those patients who did not require vasopressors (p < 0.0001). In addition, patients in the highest quartile of Ang-2 compared to the lower three quartiles required a greater number of vasopressors (p < 0.0001), suggesting that baseline plasma Ang-2 may enrich for vasoplegia and shock.
Discussion
To our knowledge, these findings represent the largest biomarker study of early measurements of plasma Ang-2 in a cohort of critically ill patients with physician-adjudicated ARDS and sepsis. Prior reports have found an association between Ang-2 and both organ failure and mortality in patients with respiratory failure [5] and an association between Ang-2 and the development of acute lung injury in smaller cohorts [3]. We present a novel finding with time-ordering data in critically ill patients with sepsis, demonstrating that higher plasma Ang-2 levels are associated with increased risk of developing ARDS, thereby strengthening the evidence for the importance of Ang-2 in the pathobiology of ARDS among critically ill patients with sepsis. The robust association between Ang-2 and 30-day mortality in patients with early sepsis, which is enhanced among a subgroup of patients with early sepsis who also have ARDS, suggests a conserved pathobiology role among heterogeneous critically ill patients. Our data support the potential role for measuring circulating Ang-2 to guide the delivery of therapeutic targets that target endothelial injury and may improve clinical outcomes in critical ill patients with sepsis, particularly those who are at risk of developing ARDS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12(11):1286–93.
Parikh SM. Targeting Tie2 and the host vascular response in sepsis. Sci Transl Med. 2016;8(335):335–9.
Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736–42.
Anderson BJ, Calfee CS, Liu KD, Reilly JP, Kangelaris KN, Shashaty MGS, Lazaar AL, Bayliffe AI, Gallop RJ, Miano TA, et al. Plasma sTNFR1 and IL8 for prognostic enrichment in sepsis trials: a prospective cohort study. Crit Care. 2019;23(1):400.
Yu WK, McNeil JB, Wickersham NE, Shaver CM, Bastarache JA, Ware LB. Angiopoietin-2 outperforms other endothelial biomarkers associated with severe acute kidney injury in patients with severe sepsis and respiratory failure. Crit Care. 2021;25(1):48.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Acknowledgements
We acknowledge Dr. Oleg Mayba for statistical advice and Drs. Jason Christie and Nuala Meyer for substantial contributions to generating the biomarker data analyzed in this manuscript.
Funding
This study was funded by NIH R35HL140026 and by Genentech, Inc.
Author information
Authors and Affiliations
Contributions
CSC, KD, CMH, KDL, MAM, KMR, KMS, and HZ contributed to study design, analysis and interpretation of data, and drafting and critical revision of manuscript. YC, AL, PS, KDW, and NW contributed to analysis and interpretation of data and critical revision of manuscript. DFC and DC contributed to study design, interpretation of data, and revision of manuscript. AG contributed to analysis and interpretation of data and revision of manuscript. AJ and KNK contributed to study design and analysis and interpretation of data. HC, SBK, SK, and MP contributed to study design and revision of manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of California, San Francisco. Subjects or a designated surrogate provided informed consent for study participation unless the subject died before their surrogate could be approached for informed consent or the subject was too ill to provide consent and a surrogate could not be identified within 28 days. In these cases, the IRB approved a waiver of consent.
Consent for publication
Not applicable.
Competing interests
YC, KD, ADG, AJ, KNK, SK, AL, KMS, KDW, NW, and HZ declare that they have no competing interests. DC, HC, DFC, AG, SBK, MP, and CMR were employed by Genentech and owned Roche stock at the time of the study. CSC declares grants and personal fees from Roche/Genentech, grants and personal fees from Bayer, personal fees from Gen1e Life Sciences, personal fees from Vasomune, grants from Quantum Leap Healthcare Collaborative, personal fees from Janssen, personal fees from Cellenkos, and personal fees from NGM Bio. CMH received consultancy fees from Spring Discovery. KDL declares consultancy agreements with AM Pharma, Biomerieux, BOA Medical, Neumora, and Seastar Medical; stock in Amgen; serving on the Editorial Boards of American Journal of Kidney Diseases, American Journal of Respiratory and Critical Care Medicine, and CJASN; advisory or leadership roles for the American Thoracic Society and NKF Scientific Advisory Board; and other interests or relationships with UpToDate. MAM declares a research grant paid to UCSF from Genentech-Roche and consulting income from Johnson & Johnson, Novartis, Pliant Therapeutics, Quantum Health, and Gilead. PS declares Personal fees from Astra-Zeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rosenberger, C.M., Wick, K.D., Zhuo, H. et al. Early plasma angiopoietin-2 is prognostic for ARDS and mortality among critically ill patients with sepsis. Crit Care 27, 234 (2023). https://doi.org/10.1186/s13054-023-04525-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04525-3