Abstract
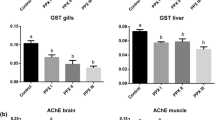
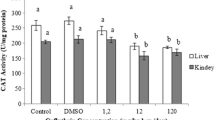
Chlorpyrifos is the most common insecticide in freshwater ecosystems, and detected in agricultural and fishery product. In this study, Oreochromis niloticus were exposed to 5, 10 and 15 ppb sublethal concentrations of chlorpyrifos in order to determine the oxidative stress response in liver. Acetylcholinesterase (AChE) activity was significantly inhibited. Superoxide dismutase activity (SOD) increased after 15 days of chlorpyrifos treatments at all concentrations (146.95%, 53.04%, 208.70%, respectively). Malondialdehyde levels were higher than that of the control level after 15 days of 5 ppb (95.65%), 10 ppb (69.56%) and 15 ppb (252.17%) chlorpyrifos treatments. Malondialdehyde levels were also increased ranging from 59.09%, 113.63% to 195.46% after 30 days of 5, 10 and 15 ppb chlorpyrifos exposures. Glutathione S-transferase activity decreased except for 15 days low concentration exposure. Catalase (CAT) activity decreased while there is no significant alteration in glutathione peroxidase activity. After recovery period, the low concentration group of chlorpyrifos provided a protection in AChE activity during recovery, but fish were observed to be unable to overcome the inhibition of AChE activity at high concentration groups. CAT activity remained reduced, SOD activity increased whereas the other biochemical parameters recovered to control levels. Results of this study suggest that chlorpyrifos induces oxidative stress in the liver of O. niloticus and this effect is not related with anti-acetylcholinesterase activity of pesticide.






Similar content being viewed by others
References
Abdullah AR, Kumar A, Chapman JC (1994) Inhibition of acetylcholinesterase in the Australian freshwater shrimp (Paratya australiensis) by profenofos. Environ Toxicol Chem 13:1861–1866
Achudume AC, Aina F, Onibere B (2010) Pollution tolerance of smoke in the distribution of neurotransmitter enzyme (acetylcholine esterase) and total cholesterol in tissues of wistar rats. J Environ Prot 1:475–479
American Public Health Association (1985) Standard methods for the examination of water and wastewater. APHA, Washington DC
Ansari BA, Kumar K (1984) Malathion toxicity: in vivo inhibition of acetylcholinesterase in the fish Brachydanio rerio (Cyprinidae). Toxicol Lett 20:283–287
Beutler E (1984) Red cell metabolism: a manual of biochemical methods. Grune and Stratton, Orlando
Brainy CDA, Saito E, Carvalho SMP, Junqueira BCV (1996) Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquat Toxicol 34:151–162
Carr RL, Ho LL, Chambers E (1997) Selective toxicity of chlorpyrifos to several species of fish during an environmental exposure: biochemical mechanisms. Environ Toxicol Chem 16:2369–2374
Dembele K, Haubruge E, Gaspar C (2000) Concentration effects of selected insecticides on brain acetylcholinesterase in the common carp (Cyprinus carpio L.). Ecotoxicol Environ Saf 45:49–54
Di Giulio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Gallagher EP, Sheehy KM (2000) Altered glutathione S-transferase catalytic activities in female brown bulheads from a contaminated central Folrida lake. Mar Environ Res 50:399–403
Giordano G, Afsharinejad Z, Guizetti M, Vitalone A, Kavanagh TJ, Costa LG (2007) Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol Appl Pharmacol 219:181–189
Habig C, Di Giulio RT (1991) Biochemical characteristics of cholinesterases in aquatic organisms. In: Mineau P (ed) Cholinesterase inhibiting insecticides. Their impact on wildlife and the environment. Elsevier, Amsterdam, p 348
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14
Hasspieler BM, Behar JV, Carlson DE, Watson DE, Di Giulio RT (1994) Susceptibility of channel catfish (Ictalurus punctatus) and brown bullhead (Ameriurus nebulosus) to oxidative stress: a comparative study. Aquat Toxicol 28:53–64
Isik I, Celik I (2008) Acute effects of methyl parathion and diazinon as inducers for oxidative stress on certain biomarkers in various tissues of rainbow trout (Onchorhynchus mykiss). Pest Biochem Physiol 92:38–42
Jin Y, Zhang X, Shu L, Chen L, Qian H (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852
Kavitha P, Venkateswara Rao J (2007) Oxidative stress and locomotor behaviour response as biomarkers for assessing recovery status of mosquito fish, Gambussia affinis after lethal effect of an organophosphate pesticide, monochrotophos. Pest Biochem Physiol 87:182–188
Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23:21–48
Kono NS, Fridowich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Kumar A, Doan H, Barnes M, Chapman KR (2010) Response and recovery of acetylcholinesterase activity in freshwater shrimp, Paratya australiensis (Decapoda: Atyidae) exposed to selected anti-cholinesterase insecticides. Ecotoxicol Environ Saf 73:1503–1510
Lackner R (1998) Oxidative stress in fish by environmental pollutants. Fish Ecotoxicol 86:203–224
Lowry OH, Rosenbrough NJ, Farr AL, Randall R (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Lushchak OV, Kubrak OI, Storey JM, Storey KB, Lushchak VI (2009) Low toxic herbicide Roundap induces mild oxidative stress in gold fish tissues. Chemosphere 76:932–937
Mansour SA, Mossa AH (2009) Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective role of zinc. Pest Biochem Physiol 93:34–39
Mansour SA, Mossa AH (2010) Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the protective role of zinc. Pest Biochem Physiol 96:14–23
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (Hemocuprein). J Biol Chem 244:6049–6055
Miron D, Pretto A, Crestani M, Glusczak L, Schetinger MR, Loro VL, Morsch VM (2008) Biochemical effects of clomazone herbicide on piavas (Lepomis obtusidens). Chemosphere 79:1–5
Morgan MJ, Fancy LL, Kiceniuk JW (1990) Response and recovery of brain acetylcholinesterase activity in Atlantic salmon (Salmo salar) exposed to fenitrothion. Can J Fish Aquat Sci 47:1652–1654
Ncibi S, Othman MB, Akacha A, Krifi MN, Zourgi L (2008) Opuntia ficus indica extract protects against chlorpyrifos-induced damage on mice liver. Food Chem Toxicol 46:797–802
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biohem 95:351–358
Oruc E, Usta D (2007) Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ Toxicol Pharmacol 23:48–55
Osterloh J, Lotti M, Pond SM (1983) Toxicological studies in a fetal overdose of 2,4-D, MCPP and chlorpyrifos. J Anal Toxicol 7:125–129
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague–Dawley rats. Environ Toxicol 24(1):66–73
Rath S, Mishra BN (1981) Toxicological effects of dichlorvos (DDVP) on brain and liver acetylcholinesterase (AChE) activity of Tilapia mossambica, Peters. Toxicology 19:239–245
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Rausiddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Sharbidre AA, Metkari V, Patode P (2011) Effect of diazinon on acetylcholinesterase activity and lipid peroxidation of Poecilia reticulata. Res J Environ Toxicol 5:152–161
Sun F, Chen SH (2008) Monitoring of pesticide chlorpyrifos residue in farmed fish: investigation of possible sources. Chemosphere 71:1866–1869
Sun F, Wong SS, Li GC, Chen SH (2006) A preliminary assessment of consumer’s exposure to pesticide residues in fisheries products. Chemosphere 62:674–680
Tridico CP, Rodrigues ACF, Nogueira L, Silva DC, Moreira AB, Almeida EA (2010) Biochemical biomarkers in Oreochromis niloticus exposed to mixtures of benzo[a]pyrene and diazinon. Ecotoxicol Environ Saf 73:858–863
Tsakaris S, Angelogianni P, Schulpis KH, Stavridis C (2000) Protective effect of l-phenylalanine on rat brain acetylcholine esterase inhibition induced by free radicals. Clin Biochem 33:103–106
Vasylkiv OY, Kubrak OI, Storey KB, Lushchak VI (2011) Catalase activity as a potential vital biomarker of fish intoxication by the herbicide aminotriazole. Pest Biochem Physiol 101:1–5
Venkataraman P, Krishnamoorthy G, Vengatesh G, Srinivasan N, Aruldhas MM, Arunakaran J (2008) Protective role of melatonin on PCB (Aroclor 1254) induced oxidative stress and changes in acetylcholine esterase and membrane bound ATPases in cerebellum, cerebral cortex and hippocampus af adult rat brain. Int J Dev Neurosci 26:585–591
Venkateswara Rao J (2006) Sublethal effects of an organophosphorus insecticide (RPR-II) on biochemical parameters of tilapia Oreochromis mossambicus. Comp Biochem Physiol 143:492–498
Xing H, Wang J, Li J, Fan Z, Wang M, Xu S (2010) Effects of atrazine and chlorpyrifos on acetylcholinesterase and carboxylesterase in brain and muscle of common carp. Environ Toxicol Pharmacol 30:26–30
Zhang JF, Wang XR, Guo HY, Wu JC, Xue YO (2004) Effects of water-soluble fractions of diesel oil on the antioxidant defences of the gold fish, Carassius auratus. Ecotoxicol Environ Saf 58:110–116
Acknowledgment
We would like to thank Cukurova University Grant Commission for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oruc, E. Oxidative Stress Responses and Recovery Patterns in the Liver of Oreochromis niloticus Exposed to Chlorpyrifos-Ethyl. Bull Environ Contam Toxicol 88, 678–684 (2012). https://doi.org/10.1007/s00128-012-0548-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0548-4