Abstract
Aims/hypothesis
Insulin resistance, a major contributor to the pathogenesis of type 2 diabetes, leads to increased hepatic glucose production (HGP) owing to an impaired ability of insulin to suppress hepatic gluconeogenesis. Nuclear receptor oestrogen-related receptor γ (ERRγ) is a major transcriptional regulator of hepatic gluconeogenesis. In this study, we investigated insulin-dependent post-translational modifications (PTMs) altering the transcriptional activity of ERRγ for the regulation of hepatic gluconeogenesis.
Methods
We examined insulin-dependent phosphorylation and subcellular localisation of ERRγ in cultured cells and in the liver of C57/BL6, leptin receptor-deficient (db/db), liver-specific insulin receptor knockout (LIRKO) and protein kinase B (PKB) β-deficient (Pkbβ −/−) mice. To demonstrate the role of ERRγ in the inhibitory action of insulin on hepatic gluconeogenesis, we carried out an insulin tolerance test in C57/BL6 mice expressing wild-type or phosphorylation-deficient mutant ERRγ.
Results
We demonstrated that insulin suppressed the transcriptional activity of ERRγ by promoting PKB/Akt-mediated phosphorylation of ERRγ at S179 and by eliciting translocation of ERRγ from the nucleus to the cytoplasm through interaction with 14-3-3, impairing its ability to promote hepatic gluconeogenesis. In addition, db/db, LIRKO and Pkbβ −/− mice displayed enhanced ERRγ transcriptional activity due to a block in PKBβ-mediated ERRγ phosphorylation during refeeding. Finally, the phosphorylation-deficient mutant ERRγ S179A was resistant to the inhibitory action of insulin on HGP.
Conclusions/interpretation
These results suggest that ERRγ is a major contributor to insulin action in maintaining hepatic glucose homeostasis.
Similar content being viewed by others
Introduction
Hepatic glucose metabolism is controlled primarily by the actions of counter-regulatory hormones: glucagon, acting through cyclic AMP (cAMP)-dependent pathways, and insulin, acting through the phosphatidylinositol 3 (PI3)-kinase pathway [1]. During fasting, glucagon contributes to the maintenance of constant levels of plasma glucose through breakdown of glycogen stored in liver via glycogenolysis and through de novo synthesis (gluconeogenesis) of additional glucose from lactate, pyruvate, glycerol and amino acids [2]. Regulation of hepatic gluconeogenesis is associated with a number of transcription factors that contribute to the transcriptional regulation of rate-limiting key gluconeogenic enzyme genes such as phosphoenolpyruvate carboxykinase (Pck1) and glucose-6-phosphatase (G6pc) [3]. During feeding, insulin mainly inhibits hepatic glucose production (HGP) through inactivation of the transcription factor, forkhead box O1 (FOXO1), by protein kinase B (PKB)-mediated phosphorylation. However, additional transcriptional regulators, including cAMP response element-binding protein (CREB)-regulated transcription coactivator 2, peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and FOXO6, have also been reported to mediate insulin inhibition of gluconeogenesis in liver; and abnormal regulation of these factors is associated with hepatic insulin resistance [4–6].
The oestrogen-related receptor (ERR) subfamily of nuclear receptors consists of three members, ERRα, ERRβ and ERRγ (NR3B1-3), which bind to classic oestrogen response elements as dimers, or to extended half-site core sequences as monomers [7]. The amino acid sequences of the three ERR isoforms are highly similar in the DNA-binding domain (DBD). In addition, the transcriptional activity of ERRs that are constitutively active in the absence of endogenous ligand depends mainly on interaction with coactivator or corepressor proteins. It is reported that the transcriptional activity of ERRγ plays an important role in the regulation of glucose, lipid, alcohol and iron metabolism in mouse liver [8, 9]. Furthermore, hepatic ERRγ expression is induced in fasting or diabetic conditions, and the increased transcriptional activity of ERRγ causes insulin resistance and glucose intolerance through induction of hepatic gluconeogenesis [10, 11]. In addition, induction of ERRγ in liver leads to impaired insulin signalling through diacylglycerol-mediated protein kinase ε activation [12], suggesting that ERRγ transcriptional activity could be involved in insulin action to maintain glucose homeostasis. In the current study, we investigated insulin-dependent post-translational modification (PTM) altering the transcriptional activity of ERRγ in the regulation of hepatic gluconeogenesis.
Methods
Animal experiments
Animal experiments were performed using 8-week-old male C57BL/6J, db/db, liver-specific insulin receptor knockout (LIRKO) and Pkbβ −/−(also known as Akt2 −/−) mice. C57BL/6J and db/db mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and liver and serum of LIRKO and Pkbβ −/−mice were provided by Dr. S. B. Biddinger (Harvard Medical School, Boston, MA, USA) and Dr. B. A. Hemmings (Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland) as described previously [13, 14]. All mice were acclimatised to a 12-h light/dark cycle at 22 ± 2°C for 2 weeks with free access to food and water in a specific pathogen-free facility. To identify the effect of insulin on ERRγ-mediated induction of hepatic gluconeogenesis in vivo, recombinant adenoviruses (Ad-GFP, Ad-Flag-ERRγ and Ad-Flag-S179A; 1 × 109 plaque-forming units) were delivered by tail-vein injection into 8-week-old male C57BL/6J mice. Plasma glucose levels were determined with an automated blood chemistry analyser (Hitachi 7150; Tokyo, Japan) and plasma insulin concentrations were measured using a mouse insulin ELISA kit (ALPCO Diagnostics, Salem, NH, USA). All animal experiments were approved and performed by the Institutional Animal Use and Care Committee of the Chonnam National University.
Analytical methods
Chemicals and DNA constructions are described in the electronic supplementary material (ESM) Methods. Rabbit anti-phospho-ERRγ was generated as described previously [15]. See ESM Methods for further details. The in vitro kinase assay was performed as described previously [16]. See ESM Methods for further details. For western blot analysis, whole-cell extracts were prepared using radioimmunoprecipitation assay (RIPA) buffer (Elpis-Biotech, Daejeon, Korea) with protease inhibitor cocktail set III (Calbiochem, San Diego, CA, USA) and phosphatase inhibitor cocktail set III (Calbiochem). See ESM Methods for further details. The glucose output assay was performed as described previously [10]. The chromatin immunoprecipitation (ChIP) assay was performed according to the manufacturer’s protocol (Millipore, Billerica, MA, USA). See ESM Methods for further details. Primary hepatocytes were isolated from Sprague-Dawley rats (male, 180–300 g, Samtako BioKorea, Seoul, Korea) by collagenase perfusion, as described previously [10]. For transient transfection assays, HepG2 (human hepatoblastoma-derived cells), AML12 (alpha mouse liver 12, a non-transformed mouse liver cell line) and 293T cells were maintained as described previously [10]. See ESM Methods for further details. Immunofluorescence staining was performed according to the manufacturer’s protocol for Alexa Fluor SFX kits (Molecular Probes, Invitrogen, Carlsbad, CA USA). See ESM Methods for further details. Quantitative PCR was performed as described previously [10]. See ESM Methods for further details.
Statistical analyses
All values are expressed as means ± SEM. The significance between mean values was evaluated by two-tailed unpaired Student’s t test.
Results
Insulin inhibits ERRγ transcriptional activity via PKB-mediated phosphorylation at S179
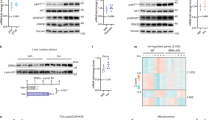
Since ERRα and ERRγ are associated with hepatic glucose metabolism, we first tested if insulin affects the transcriptional activity of ERRα and ERRγ on reporter construct sft4-luc containing three copies of ERR-binding sites (TCAAGGTTG). This reporter is driven by the promoter of the gene encoding orphan nuclear receptor small heterodimer partner (SHP), which is a known target of both ERRα and ERRγ [17]. Interestingly, transcriptional activity of ERRγ, but not of ERRα, on sft4-luc was significantly decreased by cotransfection of constitutively active PKB (PKB ca) into 293T cells (Fig. 1a). In addition, treatment with wortmannin, a PI3-kinase inhibitor, significantly restored the insulin-mediated inhibition of ERRγ transcriptional activity, whereas treatment with PD98059, a mitogen-activated protein kinase inhibitor, did not significantly change the inhibitory effect of insulin on ERRγ (Fig. 1b). These results raised the possibility that PKB could modulate the transcriptional activity of ERRγ in response to insulin. Consistent with this idea, the primary structure of ERRγ revealed the presence of a potential PKB phosphorylation site in a DBD domain of ERRγ (Fig. 1c), which was highly conserved among species from fish to mammals (ESM Fig. 1a). However, no PKB phosphorylation site was found in ERRα (Fig. 1c). Next, to test if the site is involved in PKB-mediated phosphorylation, we generated ERRγ S179A (a Ser-to-Ala substitution), a phosphorylation-deficient mutant, and ERRγ S179D (a Ser-to-Asp substitution), a phospho-mimic mutant, and found that their levels were comparable with that of wild-type (WT) ERRγ (ERRγ wt), as judged by western blot analysis (ESM Fig. 1b). As expected, basal transcriptional activity of ERRγ S179D was markedly decreased compared with that of ERRγ wt, whereas basal ERRγ S179A activity was considerably higher than that of ERRγ wt and was unaffected by PKB ca (Fig. 1d and ESM Fig. 1c). These results suggest that S179 of ERRγ plays an important role in the insulin-mediated regulation of ERRγ transcriptional activity.
Insulin inhibits ERRγ activity through PKB-mediated phosphorylation at S179. (a) PKB effect on ERRγ transactivity in 293T cells (shown as luciferase activity, sft4-luc) (+, 200 ng); *p < 0.05 vs ERRγ. (b) Effect of insulin on ERRγ transactivity in 293T cells (+, 100 ng) exposed to wortmannin (100 nmol/l) or PD98059 (10 μmol/l) for 24 h prior to 18 h treatment with insulin (100 nmol/l); *p < 0.05 and **p < 0.01 as shown. (c) Alignment of amino acid residues flanking a putative phosphorylation site of ERRα and ERRγ. The asterisk shows a substitution. (d) PKB effect on ERRγ S179A transactivity in 293T cells (+, 300 ng); *p < 0.05 vs ERRγ. (e) Domain mapping of ERRγ interacting with PKB in 293T cells. The cell lysates were immunoprecipitated with anti-FLAG. (f) In vitro PKB kinase assay. (g) Transient transfection assay showing insulin-mediated ERRγ phosphorylation in 293T cells. Insulin (100 nmol/l) was added for 18 h. (h) Effect of PKBβ knockdown on ERRγ phosphorylation in AML12 cells treated with insulin (100 nmol/l) for 18 h. Data are presented as means ± SEM. The experiment was repeated on a minimum of three separate occasions
Next, we examined the interaction between ERRγ and PKB in 293T cells, and found that ERRγ, but not ERRα, strongly interacted with PKB through the DBD domain containing the putative phosphorylation site (Fig. 1e and ESM Fig. 1d). Moreover, an in vitro kinase assay with γ-[32P] showed that active PKB directly phosphorylated ERRγ wt, but not ERRγ S179A and ERRα (Fig. 1f), indicating that ERRγ is a direct substrate of PKB. To further demonstrate PKB-mediated phosphorylation of ERRγ, we generated a phospho-specific antibody that can detect the phosphorylated form at ERRγ S179, and examined insulin-mediated phosphorylation of ERRγ in 293T cells. As expected, insulin increased ERRγ phosphorylation, but not ERRγ S179A (Fig. 1g). In addition, the ERRγ phosphorylation by insulin was induced in a time-dependent manner (ESM Fig. 1e). We also examined the effect of an ERRγ-specific inverse agonist, GSK5182, on insulin-dependent phosphorylation of ERRγ in 293T cells, and found that GSK5182 did not significantly affect the ERRγ phosphorylation levels upon insulin treatment (ESM Fig. 1f). Finally, knockdown of PKBβ expression, a major PKB isoform in liver, almost completely abolished the insulin-mediated ERRγ phosphorylation (Fig. 1h). We conclude that ERRγ transcriptional activity is inhibited by PKB phosphorylation at S179 in response to insulin.
ERRγ phosphorylation is involved in insulin receptor signalling during refeeding
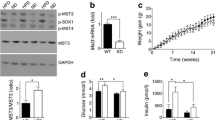
To examine the effect of insulin on ERRγ phosphorylation in vivo, we performed intraperitoneal injections of insulin into C57/BL6 mice fasted for 6 h. Hepatic ERRγ phosphorylation after the insulin injection was gradually increased until 2 h, which was consistent with the activation of insulin receptor β (IRβ) and PKB, downstream effectors of insulin (Fig. 2a), while blood glucose levels were gradually reduced after the insulin injection (ESM Fig. 2a). Since insulin is a major pancreatic hormone regulating hepatic glucose metabolism during the fed state, we next investigated whether hepatic ERRγ phosphorylation is induced by refeeding. Indeed, refeeding after 6 h of fasting led to marked induction of ERRγ phosphorylation and activation of IRβ, IRS-1 and PKB inhibiting hepatic gluconeogenesis in mice (Fig. 2b). On the other hand, db/db mice, an insulin-resistant diabetic model, did not display elevated phosphorylation of ERRγ and activation of insulin receptor signalling in liver during refeeding (Fig. 2c), resulting in increased blood glucose levels and hepatic gluconeogenic gene expression (ESM Fig. 2b, c). To further demonstrate insulin-mediated ERRγ phosphorylation in vivo, we employed LIRKO and Pkbβ −/−mice. Consistent with the results from db/db mice, enhanced ERRγ phosphorylation shown during refeeding in WT mice was abolished in both LIRKO and Pkbβ −/− mice (Fig. 2d, e). LIRKO and Pkbβ −/− mice exhibited hyperinsulinaemia, hyperglycaemia and elevated hepatic gluconeogenic gene expression during refeeding compared with WT mice (ESM Fig. 3). These results suggest that insulin-mediated ERRγ phosphorylation is operative in vivo.
ERRγ is phosphorylated through insulin receptor signalling during refeeding. (a) Western blot analysis showing phospho- and total ERRγ, IRβ and PKB protein levels in liver. C57/BL6 mice (n = 3 per group) were fed ad libitum, fasted for 6 h, or fasted for 6 h and then injected with insulin (1 U/kg). (b) Western blot analysis showing phospho- and total ERRγ, IRS-1 and PKB protein levels in liver of ad libitum fed, fasted and refed C57/BL6 mice (n = 3 per group). (c) Western blot analysis showing phospho- and total ERRγ, IRS-1 and PKB protein levels in liver of WT (n = 3) and db/db (n = 4) mice fasted for 6 h, or fasted for 6 h and then refed for 2 h. (d) Western blot analysis showing phospho- and total ERRγ protein levels in liver of WT and LIRKO mice fasted for 6 h, or fasted for 6 h and then refed for 2 h (n = 4 per group). (e) Western blot analysis showing phospho- and total ERRγ protein levels in liver of WT (n = 4) and PKBβ −/− (n = 7) mice fasted for 6 h, or fasted for 6 h and then refed for 2 h
Insulin-mediated ERRγ phosphorylation leads to its nuclear export
To explore the mechanisms underlying the inhibitory effect of insulin on the transcriptional activity of ERRγ, we examined whether insulin-mediated ERRγ phosphorylation leads to a change in subcellular localisation. Surprisingly, ERRγ wt, which was mainly localised to the nucleus without insulin stimulation, was rapidly translocated to the cytoplasm of 293T cells upon insulin treatment (Fig. 3a). However, this translocation was not observed for ERRγ S179A. To verify the cell fractionation findings, we conducted immunocytochemistry (ICC) in HepG2 cells transfected with ERRγ wt, ERRγ S179A and ERRα, and found that all of them were exclusively localised in the nucleus before insulin stimulation (Fig. 3b). However, only ERRγ wt was translocated to the cytoplasm after exposure to insulin, recapitulating the cell fractionation findings. To investigate the mechanism regarding the insulin-dependent subcellular redistribution of ERRγ, we examined the association of ERRγ with 14-3-3, a phosphorylation-dependent scaffold protein involved in subcellular redistribution [18]. A co-immunoprecipitation study performed in 293T cells indicated that 14-3-3 strongly interacted with glutathione S-transferase (GST)-conjugated ERRγ wt in the presence of insulin, but not with GST itself or ERRγ S179A (Fig. 3c). Consistent with these results, an ICC study performed in HepG2 cells demonstrated that ERRγ wt was co-localised with 14-3-3 in the cytoplasm after insulin stimulation, which was not observed for ERRγ S179A (Fig. 3d). In addition, a molecular docking model of ERRγ-DBD to double-stranded DNA (dsDNA) showed that S179 of ERRγ was located near the phosphate moieties of the bound dsDNA, causing a charge repulsion between the phospho-ERRγ S179 and the phosphate moieties of dsDNA, thereby dissociating ERRγ from dsDNA (ESM Fig. 4). Furthermore, a monomeric ERRγ-ligand binding domain can be located within the dimeric 14-3-3 cleft while maintaining similar interactions between the phospho-ERRγ S179 and bovine 14-3-3 (ESM Fig. 4). These results suggest that insulin-mediated ERRγ phosphorylation elicits its cytoplasmic translocation through interaction with 14-3-3.
Insulin leads to nuclear export of ERRγ. (a) Western blot analysis showing ERRγ localisation in 293T cells. The cells were transfected with plasmids expressing Flag-ERRγ and Flag-ERRγ S179A and then treated with insulin (100 nmol/l). ERRγ was detected with anti-FLAG. (b) ICC showing subcellular localisation of ERRγ. HepG2 cells were transfected with plasmids expressing Flag-ERRγ, Flag-ERRγ S179A or Flag-ERRα, and then treated with insulin for 6 h. Scale bar, 20 μm. (c) Western blot analysis showing the interaction between phosphorylated ERRγ and 14-3-3 in 293T cells. The cells were transfected with plasmids expressing GST only, GST-ERRγ, GST-ERRγ S179A, or Flag-14-3-3, and then treated with insulin for 18 h. (d) ICC showing subcellular localisation of ERRγ, ERRγ S179A and 14-3-3 in HepG2 cells. The cells were transfected with plasmids expressing HA-14-3-3 and Flag-ERRγ or Flag-ERRγ S179A, and then treated with insulin for 6 h. Scale bar, 10 μm. The experiment was repeated on a minimum of three separate occasions
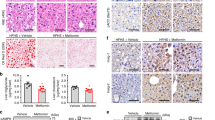
Consistent with the results in the cultured cell line, in vivo subcellular localisation analysis using immunohistochemistry (IHC) in the liver of WT mice demonstrated that ERRγ, which was exclusively localised in the nucleus during fasting, was translocated to the cytoplasm by refeeding and insulin injection (Fig. 4a, b). On the other hand, in db/db mice, it was mainly distributed in the nucleus during both fasting and refeeding. In addition, the nuclear export of ERRγ by insulin stimulation was exclusively detected in the liver of Ad-ERRγ wt mice, whereas insulin did not affect the nuclear localisation of ERRγ S179A (Fig. 4c, d). The phospho-mimic mutant ERRγ S179D showed cytoplasmic localisation. These results indicate that insulin-mediated ERRγ phosphorylation leads to its nuclear export.
Subcellular localisation of ERRγ by insulin in mice. (a) Subcellular localisation of ERRγ in the mouse liver. IHC was performed in the liver of WT (n = 3) and db/db (n = 4) mice fasted for 12 h, fasted for 6 h and then refed for 2 h, and fasted for 6 h and then injected with insulin (1 U/kg) for 2 h. IHC was performed using anti-ERRγ antibody. Scale bar, 20 μm. (b) Quantification of subcellular localisation of ERRγ; **p < 0.01, ***p < 0.001 vs WT-fasted. † p < 0.05, †† p < 0.01 vs db/db-fasted. (c) Subcellular localisation of ERRγ in the liver. C57/BL6 mice (n = 5 per group) were infected with Ad-GFP, Ad-Flag-ERRγ, Ad-Flag-ERRγ S179A or Ad-Flag-ERRγ S179D and intraperitoneally administered with insulin (1 U/kg) for 1 h after 4 h fasting at day 7 of adenoviral infections. IHC was performed using anti-ERRγ antibody. Scale bar, 20 μm. (d) Quantification of subcellular localisation of ERRγ. ***p < 0.001 vs WT. White bars, nucleus; black bars, both; grey bars, cytosol. Data are presented as means ± SEM
Insulin inhibits hepatic gluconeogenesis through inactivation of ERRγ
On the basis of the reports that ERRγ is a transcriptional regulator of hepatic gluconeogenesis [10, 11], we examined the effect of insulin on ERRγ-mediated induction of hepatic gluconeogenic gene expression. ERRγ-induced Pck1 promoter activity was significantly inhibited by co-transfection with PKB ca into 293T cells, while the phosphorylation-deficient mutant ERRγ S179A was resistant to PKB-mediated inhibition of Pck1 promoter activity (Fig. 5a). Consistent with the results in cultured cells, the infection of rat primary hepatocytes with Ad-ERRγ wt led to drastic induction of Pck1, G6pc and pyruvate dehydrogenase kinase, isoenzyme 4 (Pdk4) gene expressions compared with infection with Ad-GFP, which was significantly reduced by insulin treatment (Fig. 5b). However, insulin failed to inhibit those gene expressions induced by mutant Ad-ERRγ S179A. Interestingly, GSK5182 significantly inhibited both ERRγ wt and ERRγ S179A-induced Pck1 gene expression in AML12 cells (ESM Fig. 5). This suggests that the ERRγ inverse agonist could provide an approach to suppress the transcriptional activity of ERRγ S179A resistant to the inhibitory effect of insulin on hepatic gluconeogenesis. In addition, ChIP assays performed in rat primary hepatocytes showed that insulin disrupted the association of ERRγ wt, but not of ERRγ S179A with ERR response element (ERRE)1 and -2 of the Pck1 promoter (Fig. 5c). Furthermore, glucose output assays carried out in rat primary hepatocytes showed that insulin treatment significantly attenuated glucose output induced by forskolin or Ad-ERRγ wt, but not by Ad-ERRγ S179A (Fig. 5d). These results suggest that the inhibitory effect of insulin on hepatic gluconeogenesis depends on ERRγ inactivation through phosphorylation at S179.
ERRγ S179A is resistant to inhibition of hepatic gluconeogenesis by insulin. (a) The inhibitory effect of PKB on ERRγ wt or ERRγ S179A-mediated Pck1 promoter activity (as luciferase activity, Pck1-luc) in 293T cells transfected with the indicated plasmids; *p < 0.05 vs ERRγ. (b) Effect of PKB on ERRγ-mediated gluconeogenic gene expression in rat primary hepatocytes. Insulin (100 nmol/l) was added for 24 h. White bars, Ad-GFP; light grey bars, Ad-ERRγ; dark grey bars, Ad-ERRγ + insulin; black striped bar, Ad-S179A; black bars, Ad-S179A + insulin. *p < 0.05, **p < 0.01, ***p < 0.001 vs Ad-ERRγ. (c) ChIP assay showing occupancy of ERRγ and ERRγ S179A on Pck1 promoter. Rat primary hepatocytes were infected with Ad-Flag-ERRγ and Ad-Flag-ERRγ S179A and treated with wortmannin (100 nmol/l) for 24 h or insulin (100 nmol/l) for 18 h. Soluble chromatin was immunoprecipitated with anti-FLAG antibody. (d) Glucose output assay. Rat primary hepatocytes were infected with Ad-GFP, Ad-ERRγ or Ad-ERRγ S179A, and treated with forskolin (10 μmol/l) for 24 h or insulin for 18 h. ***p < 0.001 vs forskolin and ***p < 0.001 vs Ad-ERRγ. Data are presented as means ± SEM. The experiment was repeated on a minimum of three separate occasions
On the strength of these results, we next tested whether the mutant ERRγ S179A is resistant to the inhibitory action of insulin on hepatic gluconeogenesis in vivo. As expected, fasting blood glucose levels were significantly higher in Ad-ERRγ wt or Ad-ERRγ S179A-injected mice, compared with those of control mice (ESM Fig. 6). However, during insulin stimulation, blood glucose concentrations and hepatic gluconeogenic gene expression remained elevated in Ad-ERRγ S179A-injected mice relative to Ad-ERRγ wt-injected mice (Fig. 6a, b). Moreover, the occupancy of ERREs of Pck1 promoter by ERRγ S179A was unaffected by insulin (Fig. 6c). In addition, the phosphorylation and nuclear export of ERRγ by insulin stimulation was exclusively detected in the liver of Ad-ERRγ wt mice, but not in the liver of Ad-ERRγ S179A (Fig. 6d, e). As expected, a phospho-mimic mutant ERRγ S179D exhibited cytoplasmic localisation (Fig. 6d). Finally, glucose excursions during the insulin tolerance test were higher in Ad-ERRγ S179A-injected mice: these levels remained elevated in Ad-ERRγ S179A-injected mice compared with Ad-ERRγ wt-injected mice for up to 2 h (Fig. 6f). Taken together, these results suggest that the phosphorylation of ERRγ at S179 enables insulin, at least in part, to control glucose homeostasis in mice.
Insulin inhibits hepatic gluconeogenesis through the phosphorylation of ERRγ at S179 in mice. (a) Blood glucose levels. C57/BL6 mice (n = 5 per group) were infected with Ad-GFP (white bars), Ad-Flag-ERRγ (grey bars) or Ad-Flag-ERRγ S179A (black bars) and intraperitoneally injected with insulin (1 U/kg) for 1 h after 4 h fasting at day 7 of adenoviral infections; **p < 0.01 as shown. (b) mRNA levels of Pck1 and Pdk4 in mouse liver. ***p < 0.001 vs Ad-GFP. (c) ChIP assay showing occupancy of ERRγ and ERRγ S179A on ERREs of Pck1 promoter in mouse liver. (d) IHC showing subcellular localisation of ERRγ, ERRγ S179A and ERRγ S179D in mouse liver. IHC was performed using anti-FLAG antibody. Scale bars, 50 μm (top) and 10 μm (bottom). (e) Western blot analysis showing phospho- and total ERRγ protein levels in mouse liver. (f) Insulin tolerance test. C57/BL6 mice (n = 5 per group) infected with Ad-GFP (squares), Ad-Flag-ERRγ (circles) or Ad-Flag-ERRγ S179A (triangles) were intraperitoneally injected with 0.5 U/kg insulin after 4 h fasting. *p < 0.05 vs Ad-GFP. (g) Schematic diagram of insulin-mediated regulation of ERRγ transcriptional activity. Data are presented as means ± SEM
Discussion
In this study, we identified orphan nuclear receptor ERRγ as a novel substrate for PKB phosphorylation and demonstrated that ERRγ is a major downstream mediator of insulin action on regulation of hepatic glucose metabolism (Fig. 6g). Interestingly, we found that the PKB phosphorylation site in ERRγ is located near the second zinc finger of the DBD and its phosphorylation by insulin significantly decreases DNA-binding affinity of the receptor to the promoters of target genes. These findings are further supported by the result showing that the phospho-mimic mutant ERRγ S179D displayed significantly lower basal transcriptional activity compared with ERRγ wt. In the modelled ERRγ–DBD structure, S179 is located near the phosphate moieties of the bound dsDNA. Addition of a phosphate moiety on S179 provides a negative charge, which may detach the bound ERRγ-DBD from its cognate dsDNA due to negative charge repulsions present between the introduced phosphate group of negative charge on the S179 of ERRγ-DBD and the phosphate moieties of dsDNA. Similarly to these findings, it is reported that ERRα has four acetylation sites located in the DBD, and the acetylation changes DNA-binding and transcriptional activity [19]. Furthermore, the phosphorylation on the DBD of orphan nuclear receptor Nur77 also leads to a significant reduction of DNA-binding affinity of the receptor [20], suggesting that PTMs on DBDs of nuclear receptors could alter their transcriptional activity.
It is reported that, although ERRs possess similar functional domains and their transcriptional activity depends on interactions with similar coactivator or corepressor proteins, each ERR isoform sometimes exhibits different transcriptional outputs for the same target genes. For example, both ERRα and ERRγ can positively regulate Pdk4 expression and they have the potential to control mitochondrial programmes implicated in oxidative phosphorylation, while SHP and Lipin1 expression is regulated by ERRγ but not by ERRα or ERRβ [12, 17, 21, 22]. Moreover, PGC-1α-mediated induction of Pck1 expression was mediated by ERRγ, but inhibited by ERRα [10, 23]. In addition to the complexity of their function in the regulation of target genes, the regulation of PTMs for each ERR is also complicated. Indeed, both ERRα and ERRγ undergo PTMs such as sumoylation of the amino-terminal domain and acetylation of the DBD [19, 24], whereas ERRγ, but not ERRα, is phosphorylated by the insulin signalling pathway in this study. These results suggest that PTM of ERRs could be a key mechanism leading to alteration of their transcriptional outputs. Therefore, a specific physiological cue causing PTMs of each ERR needs to be determined.
Hormonal regulation of hepatic glucose metabolism during fasting and feeding is important for whole-body glucose homeostasis. Although the pancreatic hormone insulin is known to inhibit HGP mainly through nuclear export of FOXO1 by PKB-mediated phosphorylation, several pieces of evidence indicated that loss of Foxo1 function does not completely abolish the insulin regulation of hepatic gluconeogenesis [25–27], suggesting that additional factors are at play. Here, we demonstrated that insulin-dependent phosphorylation of ERRγ, leading to its nuclear export, is a major contributor to the inhibitory action of insulin on HGP. Moreover, a phosphorylation-deficient mutant, ERRγ S179A, is resistant to insulin action on hepatic gluconeogenesis. We also showed that elevated ERRγ phosphorylation in WT mice during feeding was not found in LIRKO or Pkbβ −/− mice, demonstrating that ERRγ is a major transcriptional mediator for maintaining the inhibitory action of insulin on hepatic gluconeogenesis. These results suggest that a defect of insulin action on ERRγ transcriptional activity may contribute to hepatic insulin resistance through an inability to phosphorylate ERRγ. In addition, generation of ERRγ S179A knock-in mice would provide further insights into the role of ERRγ in insulin action on the hepatic gluconeogenic programme. In conclusion, we propose that the inhibition of hepatic ERRγ activity would suppress the additional increase of blood glucose by the abnormal induction of hepatic gluconeogenesis in type 2 diabetes.
Abbreviations
- Ad:
-
Adenovirus
- cAMP:
-
Cyclic AMP
- ChIP:
-
Chromatin immunoprecipitation
- DBD:
-
DNA-binding domain
- dsDNA:
-
Double-stranded DNA
- ERR:
-
Oestrogen-related receptor
- ERRγ wt:
-
Wild-type ERRγ
- ERRE:
-
ERR response element
- FOXO1:
-
Forkhead box O1
- GST:
-
Glutathione S-transferase
- HGP:
-
Hepatic glucose production
- ICC:
-
Immunocytochemistry
- IHC:
-
Immunohistochemistry
- IRβ:
-
Insulin receptor β
- LIRKO:
-
Liver-specific insulin receptor knockout
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ coactivator 1-α
- PI3:
-
Phosphatidylinositol 3
- PTM:
-
Post-translational modification
- PKB:
-
Protein kinase B
- PKB ca:
-
Constitutively active PKB
- SHP:
-
Small heterodimer partner
- WT:
-
Wild-type
References
Saltiel AR (2001) New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104:517–529
Pilkis SJ, Granner DK (1992) Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 54:885–909
Yabaluri N, Bashyam MD (2010) Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci 35:473–484
Kim DH, Perdomo G, Zhang T et al (2011) FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes 60:2763–2774
Dentin R, Liu Y, Koo S-H et al (2007) Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449:366–369
Li X, Monks B, Ge Q, Birnbaum MJ (2007) Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 447:1012–1016
Tremblay AM, Giguere V (2007) The NR3B subgroup: an ovERRview. Nucl Recept Signal 5:e009
Kim DK, Kim YH, Jang HH et al (2013) Estrogen-related receptor gamma controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 62:1044–1054
Kim DK, Jeong JH, Lee JM et al (2014) Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med 20:419–424
Kim DK, Ryu D, Koh M et al (2012) Orphan nuclear receptor estrogen-related receptor gamma (ERRgamma) is key regulator of hepatic gluconeogenesis. J Biol Chem 287:21628–21639
Kim DK, Gang GT, Ryu D et al (2013) Inverse agonist of nuclear receptor ERRgamma mediates antidiabetic effect through inhibition of hepatic gluconeogenesis. Diabetes 62:3093–3102
Kim DK, Kim JR, Koh M et al (2011) Estrogen-related receptor gamma (ERRgamma) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J Biol Chem 286:38035–38042
Fisher SJ, Kahn CR (2003) Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J Clin Invest 111:463–468
Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA (2006) Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol 26:8042–8051
Woo HA, Kang SW, Kim HK, Yang KS, Chae HZ, Rhee SG (2003) Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem 278:47361–47364
Yang KJ, Shin S, Piao L et al (2008) Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem 283:1480–1491
Sanyal S, Kim JY, Kim HJ et al (2002) Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem 277:1739–1748
Tzivion G, Shen YH, Zhu J (2001) 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 20:6331–6338
Tremblay AM, Wilson BJ, Yang XJ, Giguere V (2008) Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol Endocrinol 22:570–584
Hirata Y, Kiuchi K, Chen HC, Milbrandt J, Guroff G (1993) The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J Biol Chem 268:24808–24812
Zhang Y, Ma K, Sadana P et al (2006) Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem 281:39897–39906
Dufour CR, Wilson BJ, Huss JM et al (2007) Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5:345–356
Herzog B, Cardenas J, Hall RK et al (2006) Estrogen-related receptor alpha is a repressor of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 281:99–106
Wilson BJ, Tremblay AM, Deblois G, Sylvain-Drolet G, Giguere V (2010) An acetylation switch modulates the transcriptional activity of estrogen-related receptor alpha. Mol Endocrinol 24:1349–1358
Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D (2007) Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab 6:208–216
Haeusler RA, Kaestner KH, Accili D (2010) FoxOs function synergistically to promote glucose production. J Biol Chem 285:35245–35248
Lu M, Wan M, Leavens KF et al (2012) Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 18:388–395
Acknowledgements
We would like to thank D. D. Moore (Baylor College of Medicine, Houston, TX, USA) and S.-Y. Choi (Chonnam National University Medical School, Gwangju, Republic of Korea) for critical reading and helpful discussions of the manuscript.
Funding
This work was supported by a National Creative Research Initiatives Grant (20110018305) through the National Research Foundation of Korea (NRF) funded by the Korean government (Ministry of Science, ICT & Future Planning) to H-SC; DK094162 (to SBB); and a grant of the Korea Health Technology R&D Project (A111345) funded by the Korean government (Ministry of Health & Welfare) to I-KL.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
D-KK, Y-HK, DH, YW, K-JY, DR, KSK and E-KY performed the experiments or analysis and interpretation of data. J-SK, S-HK, I-KL, H-ZC, JP, C-HL, SBB and BAH provided materials for experiments and contributed to conception and design, acquisition of data, or analysis and interpretation of data. D-KK and H-SC analysed the data and wrote the paper. All authors contributed to the discussion and revised the article and all approved the final versions of the manuscript. H-SC is responsible for the integrity of the work as a whole.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 105 kb)
ESM Figure 1
(PDF 127 kb)
ESM Figure 2
(PDF 21 kb)
ESM Figure 3
(PDF 24 kb)
ESM Figure 4
(PDF 71 kb)
ESM Figure 5
(PDF 56 kb)
ESM Figure 6
(PDF 15 kb)
Rights and permissions
About this article
Cite this article
Kim, DK., Kim, YH., Hynx, D. et al. PKB/Akt phosphorylation of ERRγ contributes to insulin-mediated inhibition of hepatic gluconeogenesis. Diabetologia 57, 2576–2585 (2014). https://doi.org/10.1007/s00125-014-3366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3366-x