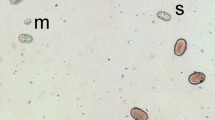
Abstract
Key message
Divergent wild and endemic peas differ in hybrid sterility in reciprocal crosses with cultivated pea depending on alleles of a nuclear ‘speciation gene’ involved in nuclear–cytoplasmic compatibility.
Background
In hybrids between cultivated and wild peas, nuclear–cytoplasmic conflict frequently occurs. One of the nuclear genes involved, Scs1, was earlier mapped on Linkage Group III.
Results
In reciprocal crosses of seven divergent pea accessions with cultivated P. sativum, some alleles of Scs1 manifested incompatibility with an alien cytoplasm as a decrease in pollen fertility to about 50 % in the heterozygotes and lack of some genotypic classes among F2 segregants. Earlier, we defined monophyletic evolutionary lineages A, B, C and D of pea according to allelic state of three markers, from nuclear, plastid and mitochondrial genomes. All tested representatives of wild peas from the lineages A and C exhibited incompatibility due to Scs1 deleterious effects in crosses with testerlines of P. sativum subsp. sativum (the common cultivated pea) at least in one direction. A wild pea from the lineage B and a cultivated pea from the lineage D were compatible with the testerline in both directions. The tested accession of cultivated P. abyssinicum (lineage A) was partially compatible in both directions. The Scs1 alleles of some pea accessions even originating from the same geographic area were remarkably different in their compatibility with cultivated Pisum sativum cytoplasm.
Conclusion
Variability of a gene involved in reproductive isolation is of important evolutionary role and nominate Scs1 as a speciation gene.


Similar content being viewed by others
References
Aksyonova E, Sinyavskaya M, Danilenko N, Pershina L, Nakamura C, Davydenko O (2005) Heteroplasmy and paternally oriented shift of the organellar DNA composition in barley–wheat hybrids during backcrosses with wheat parents. Genome 48:761–769
Anderson JA, Maan SS (1995) Interspecific nuclear–cytoplasmic compatibility controlled by genes on group 1 chromosomes in durum wheat. Genome 38:803–808
Aryamanesh N, Zeng Y, Byrne O, Hardie DC, Al-Subhi AM, Khan T, Siddique KHM, Yan G (2014) Identification of genome regions controlling cotyledon, pod wall/seed coat and pod wall resistance to pea weevil through QTL mapping. Theor Appl Genet 127:489–497
Ben-Ze’ev N, Zohary D (1973) Species relationships in the genus Pisum L. Israel J Bot 22:73–91
Bogdanova VS (2007) Inheritance of organelle DNA markers in a pea cross associated with nuclear–cytoplasmic incompatibility. Theor Appl Genet 114:333–339
Bogdanova VS, Berdnikov VA (2001) Observation of the phenomenon resembling hybrid dysgenesis in a wild pea subspecies Pisum sativum ssp. elatius. Pisum Genetics 33:5–8
Bogdanova VS, Kosterin OE (2006) A case of anomalous chloroplast inheritance in crosses of garden pea involving an accession of wild subspecies. Dokl Biol Sci 406:44–46
Bogdanova VS, Galieva ER, Kosterin OE (2009) Genetic analysis of nuclear–cytoplasmic incompatibility in pea associated with cytoplasm of an accession of wild subspecies Pisum sativum subsp. elatius (Bieb.) Schmahl. Theor Appl Genet 118:801–809
Bogdanova VS, Galieva ER, Yadrikhinskiy AK, Kosterin OE (2012) Inheritance and genetic mapping of two nuclear genes involved in nuclear–cytoplasmic incompatibility in peas (Pisum sativum L.). Theor Appl Genet 124:1503–1512
Byrne OM, Hardie DC, Khan TN, Speijers J, Yan G (2008) Genetic analysis of pod and seed resistance to pea weevil in a Pisum sativum × P. fulvum interspecific cross. Aust J Agric Res 59:854–862
Chase CD (2007) Cytoplasmic male sterility: a window to the world of plant mitochondrial–nuclear interactions. Trends Genet 23:81–90
Chen J, Ding J, Ouyang Y, Du H, Yang J, Cheng K, Zhao J, Qiu S, Zhang X, Yao J, Liu K, Wang L, Xu C, Li X, Xue Y, Xia M, Ji Q, Lu J, Xu M, Zhang Q (2008) A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proc Natl Acad Sci USA 105:11436–11441
Clement SL, Hardie DC, Elberson LR (2002) Variation among accessions of Pisum fulvum for resistance to pea weevil. Crop Sci 42:2167–2173
Fernie AR, Tadmor Y, Zamir D (2006) Natural genetic variation for improving crop quality. Curr Opin Plant Biol 9:196–202
Fondevilla S, Torres AM, Moreno MT, Rubiales D (2007) Identification of a new gene for resistance to powdery mildew in Pisum fulvum, a wild relative of pea. Breed Sci 57:181–184
Fondevilla S, Küster H, Krajinski F, Cubero JI, Rubiales D (2011) Identification of genes differentially expressed in a resistant reaction to Mycosphaerella pinodes in pea using microarray technology. BMC Genomics 12:28
Govorov LI (1937) Pisum Tourn. In: Vulf EV (ed) Kul’turnaya flora SSSR [Cultivated Flora of the USSR], vol 4. State Press of Sovhoz and Kolkhoz Literatre, Moscow, pp 229–336 (in Russian)
Greiner S, Rauwolf U, Meurer J, Herrmann RG (2011) The role of plastids in plant speciation. Mol Ecol 20:671–691
Hajjar R, HodgkinT (2007) The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156:1–13
Jing R, Ambrose MA, Knox MR, Smykal P, Hybl M, Ramos Á, Caminero C, Burstin J, Duc G, van Soest LJ, Święcicki WK, Pereira MG, Vishnyakova M, Davenport GF, Flavell AJ, Ellis TH (2012) Genetic diversity in European Pisum germplasm collections. Theor Appl Genet 125:367–380
Johnson NA (2010) Hybrid incompatibility genes: remnants of a genomic battlefield? Trends Genet 26:317–325
Kosterin OE, Bogdanova VS (2008) Relationship of wild and cultivated forms of Pisum L. as inferred from an analysis of three markers, of the plastid, mitochondrial and nuclear genomes. Genet Resour Crop Evol 55:735–755
Kosterin OE, Zaytseva OO, Bogdanova VS, Ambrose M (2010) New data on three molecular markers from different cellular genomes in Mediterranean accessions reveal new insights into phylogeography of Pisum sativum L. subsp. elatuis (Beib.) Schmahl. Genet Resour Crop Evol 57:733–739
Lu Y, Kermicle JL, Evans MM (2014) Genetic and cellular analysis of cross-incompatibility in Zea mays. Plant Reprod 27:19–29
Makasheva RKh (1979) Gorokh [Pea]. In: Korovina ON (ed) Kul’turnaya flora SSSR [Cultivated Flora of the USSR], vol 4., part 1, Kolos, Leningrad (in Russian), pp 1–324
Martin NH, Willis JH (2010) Geographical variation in postzygotic isolation and its genetic basis within and between two Mimulus species. Philos Trans R B365:2469–2478
Maxted N, Ambrose M (2001) Peas (Pisum L.). In: Maxted N, Bennett SJ (eds) Plant genetic resources of legumes in the Mediterranean. Current Plant Science and Biotechnology in Agriculture, vol 39. Kluwer Academic Press, Dordrecht, pp 181–190
McCouch S (2004) Diversifying selection in plant breeding. PLoS Biol 2:e347
Ohtsuka I (1991) Genetic differentiation in wheat nuclear genomes in relation to compatibility with Aegilops squarrosa cytoplasm and application to phylogeny of polyploid wheats. J Fac Agric Hokkaido Univ 65:127–198
Ouyang Y, Liu Y-G, Zhang Q (2010) Hybrid sterility in plant: stories from rice. Curr Opin Plant Biol 13:186–192
Rieseberg LH, Blackman BK (2010) Speciation genes in plants. Ann Bot 106:439–455
Sharma S, Upadhyaya HD, Varshney RK, Gowda CL (2013) Pre-breeding for diversification of primary gene pool and genetic enhancement of grain legumes. Front Plant Sci 4:309
Singh RJ (2003) Plant Cytogenetics, 2nd edn. CRC Press, Boca Raton 21
Sweigart AL, Fishman L, Willis JH (2006) A simple genetic incompatibility causes hybrid male sterility in Mimulus. Genetics 172:2465–2479
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Wroth JM (1998) Possible role for wild genotypes of Pisum spp. to enhance ascochyta blight resistance in pea. Aust J Exp Agric 38:469–479
Yadrikhinskiy AK, Bogdanova VS (2011) Nuclear–cytoplasm conflict in crosses of pea subspecies is controlled by alleles of a nuclear gene on Linkage Group III. Dokl Biol Sci 441:392–395
Zaytseva OO, Bogdanova VS, Kosterin OE (2012) Phylogenetic reconstruction at the species and intraspecies levels in the genus Pisum (L.) (peas) using a histone H1 gene. Gene 504:192–202
Acknowledgments
This work has been supported by Russian Foundation for Fundamental Research, Grant number 13-04-00516A and the project VI.53.1.3. Pollen counts were made with the use of the Centre of Microscopy of Biological Objects of ICG SB RAS.
Ethical statement
The experiments comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Xue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bogdanova, V.S., Kosterin, O.E. & Yadrikhinskiy, A.K. Wild peas vary in their cross-compatibility with cultivated pea (Pisum sativum subsp. sativum L.) depending on alleles of a nuclear–cytoplasmic incompatibility locus. Theor Appl Genet 127, 1163–1172 (2014). https://doi.org/10.1007/s00122-014-2288-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2288-9