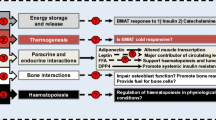
Abstract
Replacement of red hematopoietic bone marrow with yellow adipocyte-rich marrow is a conserved physiological process among mammals. The extent of this conversion is influenced by a wide array of pathological and non-pathological conditions. Of particular interest is the observation that some marrow adipocyte-inducing factors seem to oppose each other, for instance obesity and caloric restriction. Intriguingly, several important molecular characteristics of bone marrow adipose tissue (BMAT) are distinct from the classical depots of white and brown fat tissue. This depot of fat has recently emerged as an active part of the bone marrow niche that exerts paracrine and endocrine functions thereby controlling osteogenesis and hematopoiesis. While some functions of BMAT may be beneficial for metabolic adaptation and bone homeostasis, respectively, most findings assign bone fat a detrimental role during regenerative processes, such as hematopoiesis and osteogenesis. Thus, an improved understanding of the biological mechanisms leading to formation of BMAT, its molecular characteristics, and its physiological role in the bone marrow niche is warranted. Here we review the current understanding of BMAT biology and its potential implications for health and the development of pathological conditions.


Similar content being viewed by others
References
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171
Kricun ME (1985) Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skelet Radiol 14:10–19
Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A (2013) Marrow fat and bone—new perspectives. J Clin Endocrinol Metab 98:935–945
Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC (2012) Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging 36:225–230
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19:109–124
Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, Grunfeld C (2012) Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab 97:1337–1346
Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T (1987) Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8:157–164
Castro JP, Joseph LA, Shin JJ, Arora SK, Nicasio J, Shatzkes J, Raklyar I, Erlikh I, Pantone V, Bahtiyar G et al (2005) Differential effect of obesity on bone mineral density in White, Hispanic and African American women: a cross sectional study. Nutr Metab (Lond) 2:9
Doucette CR, Horowitz MC, Berry R, MacDougald OA, Anunciado-Koza R, Koza RA, Rosen CJ (2015) A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J Cell Physiol 230:2032–2037
Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Yamasaki H, Nagata Y (2000) Difference in the effect of adiposity on bone density between pre- and postmenopausal women. Maturitas 34:261–266
Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK et al (2015) Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 6:7808
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A et al (2017) Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20:771–784
Nakashima K, de Crombrugghe B (2003) Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet 19:458–466
Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM (2000) Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307
Kang S, Akerblad P, Kiviranta R, Gupta RK, Kajimura S, Griffin MJ, Min J, Baron R, Rosen ED (2012) Regulation of early adipose commitment by Zfp521. PLoS Biol 10:e1001433
Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F, Baron R (2014) Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol Cell Biol 34:3076–3085
Tavassoli M (1976) Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med 100:16–18
Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC (2005) Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging 22:279–285
Scheller EL, Rosen CJ (2014) What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci 1311:14–30
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT et al (2014) Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20:368–375
Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, Link TM (2013) Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 28:1721–1728
Berry R, Rodeheffer MS, Rosen CJ, Horowitz MC (2015) Adipose tissue residing progenitors (adipocyte lineage progenitors and adipose derived stem cells (ADSC)). Curr Molec Biol Rep 1:101–109
Berendsen AD, Olsen BR (2014) Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol Life Sci 71:493–497
Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS (2014) Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell 29:340–349
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014) Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168
Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ (2017) Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol 19:891–903
Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P (2013) Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One 8:e71318
Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T et al (2015) Identification and specification of the mouse skeletal stem cell. Cell 160:285–298
Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y et al (2015) Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160:269–284042
Berry R, Rodeheffer MS (2013) Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15:302–308
Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S et al (2012) Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab 15:230–239
Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T et al (2011) Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A 108:143–148
Arner P, Ryden M (2017) The contribution of bone marrow-derived cells to the human adipocyte pool. Adipocyte 6:1–6
Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F (2014) Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One 9:e85161
Tencerova M, Kassem M (2016) The bone marrow-derived stromal cells: commitment and regulation of adipogenesis. Front Endocrinol 7:127
Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M et al (2017) Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab 25:661–672
Sanchez-Gurmaches J, Guertin DA (2014) Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun 5:4099
Sanchez-Gurmaches J, Hsiao WY, Guertin DA (2015) Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Rep 4:541–550
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B (2012) Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 50:546–552
Liu LF, Shen WJ, Ueno M, Patel S, Azhar S, Kraemer FB (2013) Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PLoS One 8:e72367
Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ (1989) Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol 17:34–37
Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK (2011) Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 19:49–53
Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML (2010) Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res 25:2078–2088
Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A (2012) Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res 27:1864–1871
Bathija A, Davis S, Trubowitz S (1979) Bone marrow adipose tissue: response to acute starvation. Am J Hematol 6:191–198
Dietz AA, Steinberg B (1953) Chemistry of bone marrow: VIII. Composition of rabbit bone marrow in inanition. Arch Biochem Biophys 45:10–20
Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, Klibanski A (2012) Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab 97:E584–E590
Iwaniec UT, Philbrick KA, Wong CP, Gordon JL, Kahler-Quesada AM, Olson DA, Branscum AJ, Sargent JL, DeMambro VE, Rosen CJ et al (2016) Room temperature housing results in premature cancellous bone loss in growing female mice: implications for the mouse as a preclinical model for age-related bone loss. Osteoporos Int 27:3091–3101
Riggs BL, Khosla S, Melton LJ 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302
Trudel G, Payne M, Madler B, Ramachandran N, Lecompte M, Wade C, Biolo G, Blanc S, Hughson R, Bear L et al (2009) Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol (Bethesda, Md : 1985) 107:540–548
Kurabayashi T, Tomita M, Matsushita H, Honda A, Takakuwa K, Tanaka K (2001) Effects of a beta 3 adrenergic receptor agonist on bone and bone marrow adipocytes in the tibia and lumbar spine of the ovariectomized rat. Calcif Tissue Int 68:248–254
Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, Case N, Xie Z, Sen B, Romaine A et al (2014) Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 64:39–46
Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, Sen B, Xie Z, Horowitz MC, Styner MA et al (2015) Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology 156:2753–2761
Chen Y, Wang S, Bu S, Wang Y, Duan Y, Yang S (2011) Treadmill training prevents bone loss by inhibition of PPARgamma expression but not promoting of Runx2 expression in ovariectomized rats. Eur J Appl Physiol 111:1759–1767
Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA et al (2006) Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91:3349–3354
Pop LM, Lingvay I, Yuan Q, Li X, Adams-Huet B, Maalouf NM (2017) Impact of pioglitazone on bone mineral density and bone marrow fat content. Osteoporos Int. https://doi.org/10.1007/s00198-017-4164-3
Crossno JT Jr, Majka SM, Grazia T, Gill RG, Klemm DJ (2006) Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest 116:3220–3228
Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ (2009) Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology 150:1330–1340
Sulston RJ, Learman BS, Zhang B, Scheller EL, Parlee SD, Simon BR, Mori H, Bree AJ, Wallace RJ, Krishnan V et al (2016) Increased circulating adiponectin in response to thiazolidinediones: investigating the role of bone marrow adipose tissue. Front Endocrinol 7:128
Suchacki KJ, Cawthorn WP, Rosen CJ (2016) Bone marrow adipose tissue: formation, function and regulation. Curr Opin Pharmacol 28:50–56
Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55:693–698
Ralston SH, de Crombrugghe B (2006) Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev 20: 2492–2506
Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL (2017) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol 13:208–219
Botolin S, McCabe LR (2007) Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 148:198–205
Dede AD, Tournis S, Dontas I, Trovas G (2014) Type 2 diabetes mellitus and fracture risk. Metabolism 63:1480–1490
Olechnowicz SW, Edwards CM (2014) Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res 74:1625–1631
Reagan MR, Liaw L, Rosen CJ, Ghobrial IM (2015) Dynamic interplay between bone and multiple myeloma: emerging roles of the osteoblast. Bone 75:161–169
Lwin ST, Olechnowicz SW, Fowler JA, Edwards CM (2015) Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia 29:507–510
Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I (2015) Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis 32:353–368
Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, Rosen CJ, Iwaniec UT (2010) Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res Off J Am Soc Bone Miner Res 25:757–768
Lecka-Czernik B (2012) Marrow fat metabolism is linked to the systemic energy metabolism. Bone 50:534–539
Geer EB, Shen W, Strohmayer E, Post KD, Freda PU (2012) Body composition and cardiovascular risk markers after remission of Cushing’s disease: a prospective study using whole-body MRI. J Clin Endocrinol Metab 97:1702–1711
Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S (2008) Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 19:1323–1330
Tamura N, Kurabayashi T, Nagata H, Matsushita H, Yahata T, Tanaka K (2005) Effects of testosterone on cancellous bone, marrow adipocytes, and ovarian phenotype in a young female rat model of polycystic ovary syndrome. Fertil Steril 84(Suppl 2):1277–1284
Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC et al (2012) Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A 109:3143–3148
Hanks LJ, Gutierrez OM, Bamman MM, Ashraf A, McCormick KL, Casazza K (2015) Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J Clin Translat Endocrinol 2:77–82
Upadhyay J, Farr OM, Mantzoros CS (2015) The role of leptin in regulating bone metabolism. Metabolism 64:105–113
Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ (2016) Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell 18:1–15
Hamrick MW (2004) Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res 19:1607–1611
Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo XE et al (2013) Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab 17:901–915
Ma YH, Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Eiriksdottir G, Hauksdottir AM et al (2014) Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J Clin Endocrinol Metab 99:E2584–E2590
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, Mohanty ST, Croucher P, Kramer I, Kneissel M et al (2017) The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. https://doi.org/10.1002/jcp.25976
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Georgiou KR, Hui SK, Xian CJ (2012) Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells 1:205–224
Green DE, Adler BJ, Chan ME, Lennon JJ, Acerbo AS, Miller LM, Rubin CT (2013) Altered composition of bone as triggered by irradiation facilitates the rapid erosion of the matrix by both cellular and physicochemical processes. PLoS One 8:e64952
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ (2009) Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460:259–263
Botolin S, McCabe LR (2006) Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 209:967–976
Zhu RJ, Wu MQ, Li ZJ, Zhang Y, Liu KY (2013) Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int J Hematol 97:58–72
Rohrborn D, Wronkowitz N, Eckel J (2015) DPP4 in diabetes. Front Immunol 6:386
Meng J, Ma X, Wang N, Jia M, Bi L, Wang Y, Li M, Zhang H, Xue X, Hou Z et al (2016) Activation of GLP-1 receptor promotes bone marrow stromal cell osteogenic differentiation through beta-catenin. Stem Cell Rep 6:633
Duque G, Li W, Adams M, Xu S, Phipps R (2011) Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporos Int 22:1547–1553
Glorie L, Behets GJ, Baerts L, De Meester I, D’Haese PC, Verhulst A (2014) DPP IV inhibitor treatment attenuates bone loss and improves mechanical bone strength in male diabetic rats. Am J Phys Endocrinol Metab 307: E447–E455
Laharrague P, Larrouy D, Fontanilles AM, Truel N, Campfield A, Tenenbaum R, Galitzky J, Corberand JX, Penicaud L, Casteilla L (1998) High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J 12:747–752
Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC (2008) Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 118:2132–2147
Karsenty G, Ferron M (2012) The contribution of bone to whole-organism physiology. Nature 481:314–320
Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, Karsenty G (2014) Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124:1–13
Napoli N, Strollo R, Paladini A, Briganti SI, Pozzilli P, Epstein S (2014) The alliance of mesenchymal stem cells, bone, and diabetes. Int J Endocrinol 2014:690783
Singh S, Kumar D, Lal AK (2015) Serum osteocalcin as a diagnostic biomarker for primary osteoporosis in women. J Clin Diagn Res 9:Rc04–Rc07
Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J et al (2017) MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543:385–390
Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B et al (2010) Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 59:872–882
Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X (2010) Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59:1376–1385
Broxmeyer HE, Hoggatt J, O’Leary HA, Mantel C, Chitteti BR, Cooper S, Messina-Graham S, Hangoc G, Farag S, Rohrabaugh SL et al (2012) Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med 18:1786–1796
Kim JE, Ahn MW, Baek SH, Lee IK, Kim YW, Kim JY, Dan JM, Park SY (2008) AMPK activator, AICAR, inhibits palmitate-induced apoptosis in osteoblast. Bone 43:394–404
Gasparrini M, Rivas D, Elbaz A, Duque G (2009) Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp Gerontol 44:613–618
Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ (2000) Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone 26:485–489
Elbaz A, Wu X, Rivas D, Gimble JM, Duque G (2010) Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med 14:982–991
Gunaratnam K, Vidal C, Boadle R, Thekkedam C, Duque G (2013) Mechanisms of palmitate-induced cell death in human osteoblasts. Biol Open 2:1382–1389
Bermeo S, Gunaratnam K, Duque G (2014) Fat and bone interactions. Curr Osteoporos Rep 12:235–242
Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG (2000) Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology 217:527–538
Kim SW, Cho EH (2016) High levels of serum DPP-4 activity are associated with low bone mineral density in obese postmenopausal women. Endocrinol Metab 31:93–99
Dombrowski S, Kostev K, Jacob L (2017) Use of dipeptidyl peptidase-4 inhibitors and risk of bone fracture in patients with type 2 diabetes in Germany—a retrospective analysis of real-world data. Osteoporos Int 28:2421–2428
Devlin MJ, Van Vliet M, Motyl K, Karim L, Brooks DJ, Louis L, Conlon C, Rosen CJ, Bouxsein ML (2014) Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology 155:3806–3816
Iwaniec UT, Turner RT (2013) Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone 53:145–153
Keune JA, Wong CP, Branscum AJ, Iwaniec UT, Turner RT (2017) Bone marrow adipose tissue deficiency increases disuse-induced bone loss in male mice. Sci Rep 7:46325
Asada N, Takeishi S, Frenette PS (2017) Complexity of bone marrow hematopoietic stem cell niche. Int J Hematol 106:45–54
Adler BJ, Kaushansky K, Rubin CT (2014) Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol 10:737–748
Trottier MD, Naaz A, Li Y, Fraker PJ (2012) Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A 109:7622–7629
Claycombe K, King LE, Fraker PJ (2008) A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci U S A 105:2017–2021
Luo Y, Chen GL, Hannemann N, Ipseiz N, Kronke G, Bauerle T, Munos L, Wirtz S, Schett G, Bozec A (2015) Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab 22:886–894
Tang D, Tao S, Chen Z, Koliesnik IO, Calmes PG, Hoerr V, Han B, Gebert N, Zornig M, Loffler B et al (2016) Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J Exp Med 213:535–553
Spindler TJ, Tseng AW, Zhou X, Adams GB (2014) Adipocytic cells augment the support of primitive hematopoietic cells in vitro but have no effect in the bone marrow niche under homeostatic conditions. Stem Cells Dev 23:434–441
Chitteti BR, Cheng YH, Poteat B, Rodriguez-Rodriguez S, Goebel WS, Carlesso N, Kacena MA, Srour EF (2010) Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood 115:3239–3248
Sulston RJ, Cawthorn WP (2016) Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Invest 28:21–38
Liu Y (2006) Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis 9:230–234
Meynet O, Ricci JE (2014) Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med 20:419–427
Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA (2016) Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab 27:392–403
Funding
This work was supported by the European Research Council (ERC-StG 311082), the Emmy Noether Program of the German Research Foundation (DFG, grant SCHU 2445/2-1), and a grant from the German Ministry of Education and Research (BMBF) and the State of Brandenburg (DZD grant 82DZD00302).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambrosi, T.H., Schulz, T.J. The emerging role of bone marrow adipose tissue in bone health and dysfunction. J Mol Med 95, 1291–1301 (2017). https://doi.org/10.1007/s00109-017-1604-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1604-7