Abstract
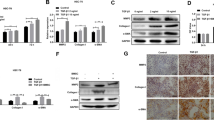
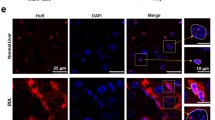
Sphingosine 1-phosphate (S1P) participates in migration of bone marrow (BM)-derived mesenchymal stem cells (BMSCs) toward damaged liver via upregulation of S1P receptor 3 (S1PR3) during mouse liver fibrogenesis. But, the molecular mechanism is still unclear. HuR, as an RNA-binding protein, regulates tumor cell motility. Here, we examined the role of HuR in migration of human BMSCs (hBMSCs) in liver fibrosis. Results showed that HuR messenger RNA (mRNA) level was increased in human or mouse fibrotic livers, and correlated with S1PR3 mRNA expression. Using immunofluorescence, we found that HuR mainly localized in the nuclei of hepatocytes and non-parenchymal cells in normal livers. However, in fibrotic livers, we detected an increased HuR cytoplasmic localization in non-parenchymal cells. In chimeric mice of BM cell-labeled by EGFP, significant numbers of EGFP-positive cells (BM origin) were positive for HuR in fibrotic areas. Meanwhile, HuR-positive cells were also positive for α-SMA (myofibroblasts). In vitro, S1P induced hBMSCs migration via S1PR3 upregulation. HuR involved in S1P-induced hBMSCs migration and increased stabilization of S1PR3 mRNA via competing with miR-30e. RNA immunoprecipitation showed that HuR interacted with S1PR3 mRNA 3′UTR. Moreover, S1P resulted in phosphorylation and cytoplasmic translocation of HuR via S1PR3 and p38MAPK. Furthermore, we transplanted EGFP+ BMSCs with or without HuR small interfering RNA (siRNA) into carbon tetrachloride-treated mice and found that knockdown of HuR inhibited the migration of BMSCs toward injured livers by flow cytometric analysis in vivo. We identified a positive feedback regulation mechanism between HuR and S1PR3 in S1P-induced BMSCs migration. HuR participates in upregulation of S1PR3 induced by S1P. S1P results in phosphorylation and translocation of HuR via S1PR3. Our results provide a new regulatory manner to the mechanism of liver fibrogenesis.
Key message
-
HuR expression and cytoplasmic localization were increased in fibrotic livers.
-
S1P induced migration of human bone marrow Mesenchymal Stem Cells via S1PR3 and HuR.
-
HuR regulated S1PR3 mRNA expression by binding with S1PR3 mRNA 3’UTR.
-
S1P induced HuR phosphorylation and cytoplasmic translocation via S1PR3.
-
HuR regulated S1PR3 expression by competing with miR-30e.







Similar content being viewed by others
References
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218
Friedman SL (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669
Iwaisako K, Brenner DA, Kisseleva T (2012) What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol 27(Suppl 2):65–68
Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou Gharios G, Jeffery R, Iredale JP, Forbes SJ (2006) The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130:1807–1821
Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L, Li L (2009) Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol 50:1174–1183
Yang L, Chang N, Liu X, Han Z, Zhu T, Li C, Yang L, Li L (2012) Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-beta1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J Pathol 181:85–97
Schwalm S, Pfeilschifter J, Huwiler A (2013) Sphingosine-1-phosphate: a Janus-Faced Mediator of fibrotic diseases. Biochim Biophys Acta 1831:239–250
Takuwa Y, Ikeda H, Okamoto Y, Takuwa N, Yoshioka K (2013) Sphingosine-1-phosphate as a mediator involved in development of fibrotic diseases. Biochim Biophys Acta 1831:185–192
Pyne NJ, Dubois G, Pyne S (2013) Role of sphingosine 1-phosphate and lysophosphatidic acid in fibrosis. Biochim Biophys Acta 1831:228–238
Sic H, Kraus H, Madl J, Flittner KA, von Munchow AL, Pieper K, Rizzi M, Kienzler AK, Ayata K, Rauer S, et al. (2014) Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J Allergy Clin Immunol 134:420–428
Mahajan-Thakur S, Sostmann BD, Fender AC, Behrendt D, Felix SB, Schror K, Rauch BH (2014) Sphingosine-1-phosphate induces thrombin receptor PAR-4 expression to enhance cell migration and COX-2 formation in human monocytes. J Leukoc Biol 96:611–618
Li C, Jiang X, Yang L, Liu X, Yue S, Li L (2009) Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol 175:1464–1472
Liu X, Yue S, Li C, Yang L, You H, Li L (2011) Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation. J Cell Physiol 226:2370–2377
Li C, Zheng S, You H, Liu X, Lin M, Yang L, Li L (2011) Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J Hepatol 54:1205–1213
Sanchez T, Hla T (2004) Structural and functional characteristics of S1P receptors. J Cell Biochem 92:913–922
Weichand B, Weis N, Weigert A, Grossmann N, Levkau B, Brüne B (2013) Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur J Immunol 43:3306–3313
Koch A, Völzke A, Puff B, Blankenbach K, Meyer Zu Heringdorf D, Huwiler A, Pfeilschifter J (2013) PPARγ agonists upregulate sphingosine 1-phosphate (S1P) receptor 1 expression, which in turn reduces S1P-induced [Ca2+]i increases in renal mesangial cells. Biochimica et Biophys Acta (BBA) - Mol Cell Biol Lipids 1831:1634–1643
Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T (2007) Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117:2506–2516
Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P (2010) Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol Biol Cell 21:1111–1124
Chang N, Xiu L, Li L (2014) Sphingosine 1-phosphate receptors negatively regulate collagen type I/III expression in human bone marrow-derived mesenchymal stem cell. J Cell Biochem 115:359–367
Simone LE, Keene JD (2013) Mechanisms coordinating ELAV/Hu mRNA regulons. Curr Opin Genet Dev 23:35–43
Srikantan S, Gorospe M (2012) HuR function in disease. Front Biosci (Landmark Ed) 17:189–205
Woodhoo A, Iruarrizaga-Lejarreta M, Beraza N, Garcia-Rodriguez JL, Embade N, Fernandez-Ramos D, Martinez-Lopez N, Gutierrez-De JV, Arteta B, Caballeria J, et al. (2012) Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology 56:1870–1882
Bai D, Gao Q, Li C, Ge L, Gao Y, Wang H (2012) A conserved TGFbeta1/HuR feedback circuit regulates the fibrogenic response in fibroblasts. Cell Signal 24:1426–1432
Atasoy U, Watson J, Patel D, Keene JD (1998) ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J Cell Sci 111(Pt 21):3145–3156
Johann AM, Weigert A, Eberhardt W, Kuhn AM, Barra V, von Knethen A, Pfeilschifter JM, Brune B (2008) Apoptotic cell-derived sphingosine-1-phosphate promotes HuR-dependent cyclooxygenase-2 mRNA stabilization and protein expression. J Immunol 180:1239–1248
Doller A, Schlepckow K, Schwalbe H, Pfeilschifter J, Eberhardt W (2010) Tandem phosphorylation of serines 221 and 318 by protein kinase Cdelta coordinates mRNA binding and nucleocytoplasmic shuttling of HuR. Mol Cell Biol 30:1397–1410
Kinoshita E (2005) Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics 5:749–757
Ge J, Chang N, Zhao Z, Tian L, Duan X, Yang L, Li L (2016) Essential roles of RNA-binding protein HuR in activation of hepatic stellate cells induced by transforming growth factor-beta1. Sci Rep 6:22141
Yi J, Chang N, Liu X, Guo G, Xue L, Tong T, Gorospe M, Wang W (2010) Reduced nuclear export of HuR mRNA by HuR is linked to the loss of HuR in replicative senescence. Nucleic Acids Res 38:1547–1558
Srikantan S, Tominaga K, Gorospe M (2012) Functional interplay between RNA-binding protein HuR and microRNAs. Curr Protein Pept Sci 13:372–379
Eberhardt W, Doller A, Pfeilschifter J (2012) Regulation of the mRNA-binding protein HuR by posttranslational modification: spotlight on phosphorylation. Curr Protein Pept Sci 13:380–390
Doller A, Pfeilschifter J, Eberhardt W (2008) Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20:2165–2173
Doller A, Winkler C, Azrilian I, Schulz S, Hartmann S, Pfeilschifter J, Eberhardt W (2011) High-constitutive HuR phosphorylation at Ser 318 by PKC{delta} propagates tumor relevant functions in colon carcinoma cells. Carcinogenesis 32:676–685
Hsu CK, Lee IT, Lin CC, Hsiao LD, Yang CM (2015) Sphingosine-1-phosphate mediates COX-2 expression and PGE2 /IL-6 secretion via c-Src-dependent AP-1 activation. J Cell Physiol 230:702–715
Brinkmann V (2007) Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115:84–105
Yiakouvaki A, Dimitriou M, Karakasiliotis I, Eftychi C, Theocharis S, Kontoyiannis DL (2012) Myeloid cell expression of the RNA-binding protein HuR protects mice from pathologic inflammation and colorectal carcinogenesis. J Clin Invest 122:48–61
Guo X, Wu Y, Hartley RS (2009) MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol 6:575–583
Dormoy-Raclet V, Menard I, Clair E, Kurban G, Mazroui R, Di Marco S, von Roretz C, Pause A, Gallouzi IE (2007) The RNA-binding protein HuR promotes cell migration and cell invasion by stabilizing the beta-actin mRNA in a U-rich-element-dependent manner. Mol Cell Biol 27:5365–5380
Al-Souhibani N, Al-Ghamdi M, Al-Ahmadi W, Khabar KS (2014) Posttranscriptional control of the chemokine receptor CXCR4 expression in cancer cells. Carcinogenesis 35:1983–1992
Hinman MN, Lou H (2008) Diverse molecular functions of Hu proteins. Cell Mol Life Sci 65:3168–3181
Zhuang R, Rao JN, Zou T, Liu L, Xiao L, Cao S, Hansraj NZ, Gorospe M, Wang JY (2013) miR-195 competes with HuR to modulate stim1 mRNA stability and regulate cell migration. Nucleic Acids Res 41:7905–7919
Wang XW, Heegaard NH, Orum H (2012) MicroRNAs in liver disease. Gastroenterology 142:1431–1443
Sohn D, Peters D, Piekorz RP, Budach W, Janicke RU (2016) miR-30e controls DNA damage-induced stress responses by modulating expression of the CDK inhibitor p21WAF1/CIP1 and caspase-3. Oncotarget. doi:10.18632/oncotarget.7432
Zhang BW, Cai HF, Wei XF, Sun JJ, Lan XY, Lei CZ, Lin FP, Qi XL, Plath M, Chen H (2016) miR-30-5p Regulates Muscle Differentiation and Alternative Splicing of Muscle-Related Genes by Targeting MBNL. Int J Mol Sci 17:E182
Kwak SY, Kim BY, Ahn HJ, Yoo JO, Kim J, Bae IH, Han YH (2015) Ionizing radiation-inducible miR-30e promotes glioma cell invasion through EGFR stabilization by directly targeting CBL-B. Febs J 282:1512–1525
Jiang L, Qiu W, Zhou Y, Wen P, Fang L, Cao H, Zen K, He W, Zhang C, Dai C, Yang J (2013) A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-beta1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int 84:285–296
Ding W, Li J, Singh J, Alif R, Vazquez-Padron RI, Gomes SA, Hare JM, Shehadeh LA (2015) miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE−/− mice. Cardiovasc Res 106:131–142
Yoon JH, Abdelmohsen K, Srikantan S, Guo R, Yang X, Martindale JL, Gorospe M (2013) Tyrosine phosphorylation of HuR by JAK3 triggers dissociation and degradation of HuR target mRNAs. Nucleic Acids Res 42:1196–1208
Acknowledgments
We are grateful to Prof. Wengong Wang for providing the pcDNA-flag-HuR plasmid. This work was supported by grants from the National Natural and Science Foundation of China (31301154, 81430013), Beijing Natural Science Foundation (5144025), and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20150502).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Chang, N., Ge, J., Xiu, L. et al. HuR mediates motility of human bone marrow-derived mesenchymal stem cells triggered by sphingosine 1-phosphate in liver fibrosis. J Mol Med 95, 69–82 (2017). https://doi.org/10.1007/s00109-016-1460-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1460-x