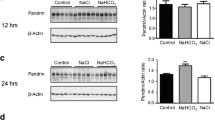
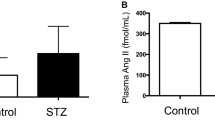
Abstract
Polycystic kidney diseases are characterized by the development of numerous bilateral renal cysts that continuously enlarge resulting in a decline of kidney function due to compression of intact nephrons. Cyst growth is driven by transepithelial chloride secretion which depends on both intracellular cAMP and calcium. Mechanisms that are involved in the regulation of the underlying secretory pathways remain incompletely understood. Here we show that glucose concentration has a strong impact on cyst growth of renal tubular cells within a collagen matrix as well as in embryonic kidneys deficient or competent for Pkd1. Glucose-dependent cyst growth correlates with the transcriptional induction of the calcium-activated chloride channel anoctamin 1 (ANO1) and its increased expression in the apical membrane of cyst-forming cells. Inhibition of ANO1 with the specific inhibitor CaCCinh-AO1 significantly decreases glucose-dependent cyst growth in both models. Ussing chamber analyses revealed increased apical chloride secretion of renal tubular cells upon exposure to high glucose medium which can also be inhibited by the use of CaCCinh-AO1. These data suggest that glycemic control may help to reduce renal cyst growth in patients with polycystic kidney disease.
Key message
-
Renal cyst growth depends on glucose concentration in two in vitro cyst models.
-
High glucose leads to upregulation of the calcium-activated chloride channel ANO1.
-
High glucose promotes calcium-activated chloride secretion via ANO1.
-
Glucose-dependent secretion can be inhibited by a specific inhibitor of ANO1.






Similar content being viewed by others
References
Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369:1287–1301
Grantham JJ, Mulamalla S, Swenson-Fields KI (2011) Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7:556–566
Terryn S, Ho A, Beauwens R, Devuyst O (2011) Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1812:1314–1321
Buchholz B, Faria D, Schley G, Schreiber R, Eckardt KU, Kunzelmann K (2014) Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst-forming epithelial cells. Kidney Int 85:1058–1067
Reed B, Helal I, McFann K, Wang W, Yan XD, Schrier RW (2012) The impact of type II diabetes mellitus in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27:2862–2865
Karalliedde J, Gnudi L (2014) Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol Dial Transplant. doi:10.1093/ndt/gfu405
Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A, Breuning MH, Verbeek S, de Heer E, Peters DJ (2006) Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis 44:225–232
Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJM (2007) Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet 16:3188–3196
Buchholz B, Teschemacher B, Schley G, Schillers H, Eckardt KU (2011) Formation of cysts by principal-like MDCK cells depends on the synergy of cAMP- and ATP-mediated fluid secretion. J Mol Med 89:251–261
Gekle M, Wünsch S, Oberleithner H, Silbernagl S (1994) Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflügers Arch 428:157–162
Buchholz B, Klanke B, Schley G, Bollag G, Tsai J, Kroening S, Yoshihara D, Wallace DP, Kraenzlin B, Gretz N et al (2011) The Raf kinase inhibitor PLX5568 slows cyst proliferation in rat polycystic kidney disease but promotes renal and hepatic fibrosis. Nephrol Dial Transplant 26:3458–3465
Schley G, Scholz H, Kraus A, Hackenbeck T, Klanke B, Willam C, Wiesener MS, Heinze E, Burzlaff N, Eckardt KU et al (2015) Hypoxia inhibits nephrogenesis through paracrine Vegfa despite the ability to enhance tubulogenesis. Kidney Int. doi:10.1038/ki.2015.214
Magenheimer BS, St John PL, Isom KS, Abrahamson DR, De Lisle RC, Wallace DP, Maser RL, Grantham JJ, Calvet JP (2006) Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+), K(+),2Cl(-) Co-transporter-dependent cystic dilation. J Am Soc Nephrol 17:3424–3437
Yang B, Sonawane ND, Zhao D, Somlo S, Verkman AS (2008) Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol 19:1300–1310
Jansson K, Nguyen AN, Magenheimer BS, Reif GA, Aramadhaka LR, Bello-Reuss E, Wallace DP, Calvet JP, Blanco G (2012) Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am J Physiol Renal Physiol 303:F982–F990
Yin L, Vijaygopal P, MacGregor GG, Menon R, Ranganathan P, Prabhakaran S, Zhang L, Zhang M, Binder HJ, Okunieff P et al (2014) Glucose stimulates calcium-activated chloride secretion in small intestinal cells. Am J Physiol Cell Physiol 306:C687–C696
Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P et al (2010) Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363:820–829
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS et al (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418
Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N et al (2010) Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363:830–840
Sas KM, Yin H, Fitzgibbon WR, Baicu CF, Zile MR, Steele SL, Amria M, Saigusa T, Funk J, Bunni MA et al (2015) Hyperglycemia in the absence of cilia accelerates cystogenesis and induces renal damage. Am J Physiol Renal Physiol 309:F79–F87
Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F et al (2013) Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19:488–493
Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Thevenod F, Roussa E, Rock J, Schreiber R (2011) Anoctamins. Pflugers Arch - Eur J Physiol. doi:10.1007/s00424-011-0975-9
Lien YH, Wang X, Gillies RJ, Martinez-Zaguilan R (1995) Modulation of intracellular Ca2+ by glucose in MDCK cells: role of endoplasmic reticulum Ca(2+)-ATPase. Am J Physiol 268:F671–F679
Tan C, Voss U, Svensson S, Erlinge D, Olde B (2013) High glucose and free fatty acids induce beta cell apoptosis via autocrine effects of ADP acting on the P2Y(13) receptor. Purinergic Signal 9:67–79
Faria D, Schreiber R, Kunzelmann K (2009) CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch - Eur J Physiol 457:1373–1380
Schreiber R, Kunzelmann K (2005) Purinergic P2Y6 receptors induce Ca2+ and CFTR dependent Cl- secretion in mouse trachea. Cell Physiol Biochem 16:99–108
Namkung W, Finkbeiner WE, Verkman AS (2010) CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell 21:2639–2648
Bruce JI, Straub SV, Yule DI (2003) Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium 34:431–444
Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Wolf L, Schreiber R (2012) Airway epithelial cells--functional links between CFTR and anoctamin dependent Cl- secretion. Int J Biochem Cell Biol 44:1897–1900
Kongsuphol P, Schreiber R, Kraidith K, Kunzelmann K (2011) CFTR induces extracellular acid sensing in Xenopus oocytes which activates endogenous Ca(2)(+)-activated Cl(-) conductance. Pflugers Arch - Eur J Physiol 462:479–487
Buchholz B, Schley G, Faria D, Kroening S, Willam C, Schreiber R, Klanke B, Burzlaff N, Jantsch J, Kunzelmann K et al (2014) Hypoxia-inducible factor-1alpha causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol 25:465–474
Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, Goldfarb M, Shina A, Zibertrest F, Eckardt KU, Rosen S et al (2008) Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73:34–42
Bondeva T, Heinzig J, Ruhe C, Wolf G (2013) Advanced glycated end-products affect HIF-transcriptional activity in renal cells. Mol Endocrinol 27:1918–1933
Faubert B, Vincent EE, Poffenberger MC, Jones RG (2015) The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett 356:165–170
Tao Y, Kim J, Schrier RW, Edelstein CL (2005) Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16:46–51
Takiar V, Nishio S, Seo-Mayer P, King JD, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ (2011) Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci 108:2462–2467
Pietrzak-Nowacka M, Safranow K, Byra E, Nowosiad M, Marchelek-Mysliwiec M, Ciechanowski K (2010) Glucose metabolism parameters during an oral glucose tolerance test in patients with autosomal dominant polycystic kidney disease. Scand J Clin Lab Invest 70:561–567
Beckles GL, Chou CF, Centers for Disease C, Prevention (2013) Diabetes - United States, 2006 and 2010. MMWR Surveill Summ 62(Suppl 3):99–104
Gardete-Correia L, Boavida JM, Raposo JF, Mesquita AC, Fona C, Carvalho R, Massano-Cardoso S (2010) First diabetes prevalence study in Portugal: PREVADIAB study. Diabet Med 27:879–881
Chang AM, Halter JB (2003) Aging and insulin secretion. Am J Physiol Endocrinol Metab 284:E7–E12
Acknowledgments
We thank Barbara Teschemacher for excellent technical support. BB was supported by the Deutsche Forschungsgemeinschaft (DFG BU2918/2-1), Else-Kroener-Fresenius Stiftung (2013_A299), the Bayerische Forschungsallianz (BayIntAn_FAU_2014_134), and the Center for Kidney and Blood Pressure Research Regensburg-Erlangen-Nuremberg (REN). GS was supported by the ELAN-Fond of the University of Erlangen-Nürnberg and REN. RS and KK were supported by SFB699 A7/A12. The present work was performed by AK in fulfillment of the requirements for obtaining the degree “Dr. rer. nat.”
Conflict of interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 191 kb)
Rights and permissions
About this article
Cite this article
Kraus, A., Schley, G., Kunzelmann, K. et al. Glucose promotes secretion-dependent renal cyst growth. J Mol Med 94, 107–117 (2016). https://doi.org/10.1007/s00109-015-1337-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1337-4