Abstract
Proline-glutamic acid (PE) and proline-proline-glutamic acid (PPE) are related proteins exclusive to Mycobacteria that play diverse roles in modulating critical innate immune pathways. In this study, we observed that the PPE57 protein is associated with the cell wall and is exposed on the cell surface. PPE57 enhances Mycobacterium spp. entering into macrophages and plays a role in macrophage phagocytosis. To explore the underlying mechanism, we demonstrated that PPE57 is able to recognise Toll-like receptor 2 (TLR2) and further induce macrophage activation by augmenting the expression of several cell surface molecules (CD40, CD80, CD86 and MHC class II) and pro-inflammatory cytokines (TNF-α, IL-6 and IL-12p40) within macrophages. These molecules are involved in the mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) signalling pathways. We demonstrated that PPE57 effectively polarises T cells to secrete interferon (IFN)-γ and IL-2 and to up-regulate CXCR3 expression in vivo and in vitro, suggesting that this protein may contribute to Th1 polarisation during the immune response. Moreover, recombinant Bacillus Calmette-Guérin (BCG) over-expressing PPE57 could provide better protective efficacy against Mycobacterium tuberculosis challenge compared with BCG. Taken together, our data provides several pieces of evidence that PPE57 may regulate innate and adaptive immunity by interacting with TLR2. These findings indicate that PPE57 protein is a potential antigen for the rational design of an efficient vaccine against M. tuberculosis.
Key messages
-
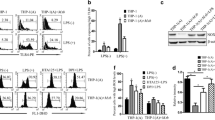
PPE57 is located on the cell surface and enhances mycobacterium entry into macrophage.
-
PPE57 interacts directly with TLR2 on macrophages.
-
PPE57 plays a key role in the activation of macrophages in a TLR2-dependent manner.
-
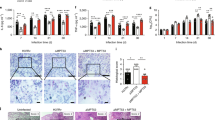
PPE57 induces a Th1 immune response via TLR2-mediated macrophage functions.
-
Recombinant BCG over-expressing PPE57 could improve protective efficacy against M. tuberculosis.










Similar content being viewed by others
References
O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP (2013) The immune response in tuberculosis. Annu Rev Immunol 31:475–527
Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A (2011) Immunological biomarkers of tuberculosis. Nat Rev Immunol 11:343–354
Smith I (2003) Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16:463–496
Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar SM (2014) In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol 12:289–299
Sanchez D, Rojas M, Hernandez I, Radzioch D, Garcia LF, Barrera LF (2010) Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis-induced macrophage death. Cell Immunol 260:128–136
Jo EK, Yang CS, Choi CH, Harding CV (2007) Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol 9:1087–1098
Lee BL, Barton GM (2014) Trafficking of endosomal Toll-like receptors. Trends Cell Biol. doi:10.1016/j.tcb.2013.12.002
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2838–2849
Fratti RA, Chua J, Deretic V (2003) Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J Biol Chem 278:46961–46967
Tapping RI, Tobias PS (2003) Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res 9:264–268
Means TK, Jones BW, Schromm AB, Shurtleff BA, Smith JA, Keane J, Golenbock DT, Vogel SN, Fenton MJ (2001) Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol 166:4074–4082
Bulut Y, Faure E, Thomas L, Equils O, Arditi M (2001) Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol 167:987–994
Ito T, Schaller M, Hogaboam CM, Standiford TJ, Sandor M, Lukacs NW, Chensue SW, Kunkel SL (2009) TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest 119:33–46
Nair S, Ramaswamy PA, Ghosh S, Joshi DC, Pathak N, Siddiqui I, Sharma P, Hasnain SE, Mande SC, Mukhopadhyay S (2009) The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J Immunol 183:6269–6281
Basu S, Pathak SK, Banerjee A, Pathak S, Bhattacharyya A, Yang Z, Talarico S, Kundu M, Basu J (2007) Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J Biol Chem 282:1039–1050
Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH (2004) Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol 173:2660–2668
Chen ST, Li JY, Zhang Y, Gao X, Cai H (2012) Recombinant MPT83 derived from Mycobacterium tuberculosis induces cytokine production and upregulates the function of mouse macrophages through TLR2. J Immunol 188:668–677
Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE (2003) The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol 170:2409–2416
Roura-Mir C, Wang L, Cheng TY, Matsunaga I, Dascher CC, Peng SL, Fenton MJ, Kirschning C, Moody DB (2005) Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J Immunol 175:1758–1766
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE et al (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544
Sampson SL (2011) Mycobacterial PE/PPE proteins at the host-pathogen interface. Clin Dev Immunol 2011, 497203. doi:10.1155/2011/497203
Le Moigne V, Robreau G, Borot C, Guesdon JL, Mahana W (2005) Expression, immunochemical characterization and localization of the Mycobacterium tuberculosis protein p27. Tuberculosis (Edinb) 85:213–219
Okkels LM, Brock I, Follmann F, Agger EM, Arend SM, Ottenhoff TH, Oftung F, Rosenkrands I, Andersen P (2003) PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect Immun 71:6116–6123
Mukhopadhyay S, Balaji KN (2011) The PE and PPE proteins of Mycobacterium tuberculosis. Tuberculosis (Edinb) 91:441–447
Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM (1999) Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520–1523
Zhang H, Wang J, Lei J, Zhang M, Yang Y, Chen Y, Wang H (2007) PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a potential B-cell antigen used for serological diagnosis to distinguish vaccinated controls from tuberculosis patients. Clin Microbiol Infect 13:139–145
Chen J, Su X, Zhang Y, Wang S, Shao L, Wu J, Wang F, Zhang S, Wang J, Weng X et al (2009) Novel recombinant RD2- and RD11-encoded Mycobacterium tuberculosis antigens are potential candidates for diagnosis of tuberculosis infections in BCG-vaccinated individuals. Microbes Infect 11:876–885
Wang J, Qie Y, Zhang H, Zhu B, Xu Y, Liu W, Chen J, Wang H (2008) PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a novel immunodominant antigen of Mycobacterium tuberculosis induces humoral and cellular immune responses in mice. Microbiol Immunol 52:224–230
Cascioferro A, Delogu G, Colone M, Sali M, Stringaro A, Arancia G, Fadda G, Palu G, Manganelli R (2007) PE is a functional domain responsible for protein translocation and localization on mycobacterial cell wall. Mol Microbiol 66:1536–1547
Dona V, Ventura M, Sali M, Cascioferro A, Provvedi R, Palu G, Delogu G, Manganelli R (2013) The PPE domain of PPE17 is responsible for its surface localization and can be used to express heterologous proteins on the mycobacterial surface. PLoS One 8:e57517
Nguyen L, Scherr N, Gatfield J, Walburger A, Pieters J, Thompson CJ (2007) Antigen 84, an effector of pleiomorphism in Mycobacterium smegmatis. J Bacteriol 189:7896–7910
Dong D, Wang D, Li M, Wang H, Yu J, Wang C, Liu J, Gao Q (2011) PPE38 modulates the innate immune response and is required for Mycobacterium marinum virulence. Infect Immun 80:43–54
Chapman R, Shephard E, Stutz H, Douglass N, Sambandamurthy V, Garcia I, Ryffel B, Jacobs W, Williamson AL (2012) Priming with a recombinant pantothenate auxotroph of Mycobacterium bovis BCG and boosting with MVA elicits HIV-1 Gag specific CD8+ T cells. PLoS One 7:e32769
Luo Y, Wang B, Hu L, Yu H, Da Z, Jiang W, Song N, Qie Y, Wang H, Tang Z et al (2009) Fusion protein Ag85B-MPT64(190-198)-Mtb8.4 has higher immunogenicity than Ag85B with capacity to boost BCG-primed immunity against Mycobacterium tuberculosis in mice. Vaccine 27:6179–6185
Sampson SL, Lukey P, Warren RM, van Helden PD, Richardson M, Everett MJ (2001) Expression, characterization and subcellular localization of the Mycobacterium tuberculosis PPE gene Rv1917c. Tuberculosis (Edinb) 81:305–317
Daleke MH, Cascioferro A, de Punder K, Ummels R, Abdallah AM, van der Wel N, Peters PJ, Luirink J, Manganelli R, Bitter W (2011) Conserved Pro-Glu (PE) and Pro-Pro-Glu (PPE) protein domains target LipY lipases of pathogenic mycobacteria to the cell surface via the ESX-5 pathway. J Biol Chem 286:19024–19034
Delogu G, Pusceddu C, Bua A, Fadda G, Brennan MJ, Zanetti S (2004) Rv1818c-encoded PE_PGRS protein of Mycobacterium tuberculosis is surface exposed and influences bacterial cell structure. Mol Microbiol 52:725–733
Brennan MJ, Delogu G (2002) The PE multigene family: a 'molecular mantra' for mycobacteria. Trends Microbiol 10:246–249
Pasare C, Medzhitov R (2005) Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol 560:11–18
Takeda K, Akira S (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14
Bansal K, Elluru SR, Narayana Y, Chaturvedi R, Patil SA, Kaveri SV, Bayry J, Balaji KN (2010) PE_PGRS antigens of Mycobacterium tuberculosis induce maturation and activation of human dendritic cells. J Immunol 184:3495–3504
Bansal K, Sinha AY, Ghorpade DS, Togarsimalemath SK, Patil SA, Kaveri SV, Balaji KN, Bayry J (2010) Src homology 3-interacting domain of Rv1917c of Mycobacterium tuberculosis induces selective maturation of human dendritic cells by regulating PI3K-MAPK-NF-kappaB signaling and drives Th2 immune responses. J Biol Chem 285:36511–36522
Saraav I, Singh S, Sharma S (2014) Outcome of Mycobacterium tuberculosis and Toll-like receptor interaction: immune response or immune evasion? Immunol Cell Biol. doi:10.1038/icb.2014.52
Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ (2002) TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 168:4620–4627
Berrington WR, Hawn TR (2007) Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev 219:167–186
Singh PP, Goyal A (2014) Interleukin-6: a potent biomarker of mycobacterial infection. Springerplus 2:686
Xu Y, Liu W, Shen H, Yan J, Yang E, Wang H (2010) Recombinant Mycobacterium bovis BCG expressing chimaeric protein of Ag85B and ESAT-6 enhances immunostimulatory activity of human macrophages. Microbes Infect 12:683–689
Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP (2004) B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 21:401–413
Howard LM, Ostrovidov S, Smith CE, Dal Canto MC, Miller SD (2002) Normal Th1 development following long-term therapeutic blockade of CD154-CD40 in experimental autoimmune encephalomyelitis. J Clin Invest 109:233–241
Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J (2007) Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8:610–618
Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B (2004) A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol 172:4733–4743
Ponnappan S, Uken-Trebilcock G, Lindquist M, Ponnappan U (2004) Tyrosine phosphorylation-dependent activation of NFkappaB is compromised in T cells from the elderly. Exp Gerontol 39:559–566
Doherty TM, Rook G (2006) Progress and hindrances in tuberculosis vaccine development. Lancet 367:947–949
Talat N, Shahid F, Perry S, Dawood G, Hussain R (2011) Th1/Th2 cytometric bead array can discriminate cytokine secretion from endogenously activated cells in pulmonary disease, recent and remote infection in tuberculosis. Cytokine 54:136–143
North RJ, Jung YJ (2004) Immunity to tuberculosis. Annu Rev Immunol 22:599–623
Hashimoto D, Nagata T, Uchijima M, Seto S, Suda T, Chida K, Miyoshi H, Nakamura H, Koide Y (2008) Intratracheal administration of third-generation lentivirus vector encoding MPT51 from Mycobacterium tuberculosis induces specific CD8+ T-cell responses in the lung. Vaccine 26:5095–5100
Williams MA, Holmes BJ, Sun JC, Bevan MJ (2006) Developing and maintaining protective CD8+ memory T cells. Immunol Rev 211:146–153
Seder RA, Darrah PA, Roederer M (2008) T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8:247–258
Mitra DK, Sharma SK, Dinda AK, Bindra MS, Madan B, Ghosh B (2005) Polarized helper T cells in tubercular pleural effusion: phenotypic identity and selective recruitment. Eur J Immunol 35:2367–2375
Saha PK, Sharma PK, Sharma SK, Singh A, Mitra DK (2013) Recruitment of Th1 effector cells in human tuberculosis: hierarchy of chemokine receptor(s) and their ligands. Cytokine 63:43–51
Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K et al (2000) Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol 68:568–574
McBride A, Konowich J, Salgame P (2013) Host defense and recruitment of Foxp3(+) T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS Pathog 9:e1003397
Williams A, Hall Y, Orme IM (2009) Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb) 89:389–397
Orme IM (2006) Preclinical testing of new vaccines for tuberculosis: a comprehensive review. Vaccine 24:2–19
Acknowledgments
This work was supported by grants from National Major Special Projects (2012ZX10003008), the NSF of China (31100660) and the NSF of Shanghai Sci. Tech. Committee (11ZR1401600).
Conflict of interest
The authors have declared that no competing interest exists.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 304 kb)
Rights and permissions
About this article
Cite this article
Xu, Y., Yang, E., Huang, Q. et al. PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2. J Mol Med 93, 645–662 (2015). https://doi.org/10.1007/s00109-014-1243-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1243-1