Abstract
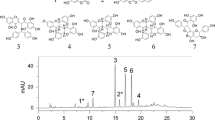
Merostachys riedeliana Rupr. is a native and overabundant bamboo species in the Brazilian Atlantic Forest. Moderate to strong allelopathic activity may be one mechanism that explains this super-dominance and the changes in structure and composition of forest areas occupied by bamboo. This study evaluated the phytotoxic effect of M. riedeliana extracts and fractions and identified their putative allelochemicals. We investigated the presence of allelochemicals in soil collected from stands occupied by M. riedeliana. Furthermore, we evaluated the putative effect of tree allelochemicals, individually and combined, on germination and growth. The aqueous extract of leaves and its ethyl acetate fraction presented the highest inhibitory effects on seed germination and seedling growth. The effect of the extracts and fractions on the target species was species-specific. Neither the individual nor the combined phenolic acids significantly inhibited seed germination; however, a pronounced growth inhibition was observed in M. bimucronata seedlings treated with vanillic acid and in E. verna and M. bimucronata seedlings treated with combined phenolic acids. Isovitexin, vitexin, isoorientin, orientin, and their O-glycoside derivatives, the lactonic dimer of the p-hydroxybenzoic acid and 3,4-methylenedioxymandelic acid were identified in the aqueous extracts and ethyl acetate fraction by Liquid Chromatography-Diode Array Dectector/Electrospray Ionization/Mass Spectrometry (LC-DAD/ESI–MS/MS). The Gas Chromatography-Mass Spectrometer (GC–MS) profile of the same extract and fraction showed the presence of benzoic, benzeneacetic, salicylic, p-hydroxybenzoic, p-hydroxyphenylacetic, vanillic, p-coumaric, protocatechuic, syringic, gallic, m-coumaric vanillylmandelic, 4-methylmandelic, 3,4-methylenedioxymandelic and trans-ferulic acids. The p-benzoic acid and the apigenin 6-C-glucoside (isovitexin) were identified in the soil extract collected from under bamboo-growing areas. Even though laboratory bioassays are not completely predictive of the allelopathic effects that occur in nature, the results of this study provide preliminary evidence of allelopathy as a possible species-specific inhibition mechanism of native species that explain the impoverishment of floristic richness and the functional groups in areas where M. riedeliana is overabundant.
Similar content being viewed by others
References
Abhilasha D, Quintana N, Vivanco J, Joshi J (2008) Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J Ecol 96:993–1001
Bauer JT, Shannon SM, Stoops RE, Reynolds HL (2012) Context dependency of the allelopathic effects of Lonicera maackii on seed germination. Plant Ecol 213:1907–1916
Bertoldi C, De Leo M, Ercoli L, Braca A (2012) Chemical profile of Festuca arundinaceae extracts showing allelochemical activity. Chemoecology 22:13–21
Blum U (2004) Fate of phenolic allelochemicals in soils—the role of the soil and rhizosphere micro-organisms. In: Maciás FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Allelopathy: chemistry and mode of action of allelopathic chemicals. CRC Press, Boca Raton, pp 57–76
Blum U (2014) Hypothetical cause and effect bioassays. Plant-Plant allelopathic interactions II. Laboratory bioassays for water-soluble compounds with an emphasis on phenolic acids. Springer, pp 237–272. doi:10.1007/978-3-319-04732-4_6
Blum U, Gerig TM (2005) Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: nutrient culture studies. J Chem Ecol 31:1907–1932
Blum U, Gerig TM (2006) Interrelationships between p-coumaric acid, evapotranspiration, soil water content, and leaf expansion. J Chem Ecol 32:1817–1834
Blum U, Dalton BR, Shann JR (1985a) Effects of various mixtures of ferulic acid and some of its microbial metabolic products on cucumber leaf expansion and dry matter in nutrient culture. J Chem Ecol 11:619–641
Blum U, Dalton BR, Shann JR (1985b) Effects of ferulic and p-coumaric acids nutrient culture of cucumber leaf expansion as influenced by ph. J Chem Ecol 11:1567–1582
Blum U, Gerig TM, Weed SB (1989) Effects of mixture of phenolic acids on leaf area expansion of cucumber seedlings growth in different pH Portsmouth A1 soil materials. J Chem Ecol 15:2413–2423
Blum U, Wentworth TR, Klein K, Worsham AD, King LD, Gerig TM, Lyu S-W (1991) Phenolic acid content of soils from wheat-no till, wheat-conventional till, and fallow-conventional till soybean cropping systems. J Chem Ecol 17:1045–1068
Blum U, Shafer SR, Lehman ME (1999) Evidence for inhibitory allelopathic interations involving phenolic acids in field soils: concepts vs. an experimental model. Crit Rev Plant Sci 18:673–693
Bogatek R, Gniazdowska A, Zakrzewska W, Oracz K, Gawronski SW (2006) Allelopathic effects of sunflower extracts on mustard seed germination and seedling growth. Biol Plant 50:156–158
Callaway RM, Maron JL (2006) What have exotic plant invasions taught us over the past 20 years? Trends Ecol Evol 21:369–374
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Campanello PI, Genoveva GM, Ares A, Montti L, Goldstein G (2007) Tree regeneration and microclimate in a liana and bamboo-dominated semideciduous Atlantic Forest. For Ecol Manag 252:108–117
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L, Renella G, Landi L, Nannipieri P (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol Fertil Soils 48:123–149
Chaves N, Sosa T, Alías JC, Escudero JC (2001) Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J Chem Ecol 27:611–621
Chou CH (2010) Role of allelopathy in sustainable agriculture: use of allelochemicals as naturally occurring bio-agrochemicals. Allelopath J 25:3–16
Chou CH, Hou MH (1981) Allelopathic researches of subtropical vegetation in Taiwan I. Evaluation of allelopathic potential of bamboo vegetation. Proc Natl Sci USA 5:14–27
Chou CH, Kuo YL (1986) Allelopathic research of subtropical vegetation in Taiwan III. Allelopathic exclusion of understory by Leucaena leucocephala (Lam.). J Chem Ecol 12:1431–1448
Chou CH, Yang CM (1982) Allelopathic research of subtropical vegetation in Taiwan II. Comparative exclusion of understory by Phyllostachys edulis and Cryptomeria japonica. J Chem Ecol 8:1489–1502
Chou CH, Fu CY, Li SY, Wang YF (1998) Allelopathic potential of Acacia confusa and related species in Taiwan. J Chem Ecol 24:2131–2150
d´Oliveira MVN, Guarino ES, Ribas LA, Acuña MHA (2013) Can forest management be sustainable in a bamboo dominated forest? A 12-year study of forest dynamics in western Amazon. For Ecol Manag 310:672–679
Dalton BR (1989) Physicochemical and biological processes affecting the recovery of exogenously applied ferulic acid from tropical forest soils. Plant Soil 115:13–22
Einhelling FA (2004) Mode of allelochemical action of phenolic compounds. In: Macías FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Allelopathy chemistry and mode of action of allelochemicals. CRC Press LLC, Boca Raton, pp 217–238
Fernandez C, Lelong B, Vila B, Mévy JP, Robles C, Greff S, Dupouyet S, Bousquet-Mélou A (2006) Potential allelopathic effect of Pinus halepensis in the secondary succession:an experimental approach. Chemoecology 16:97–105
Filgueiras TS, Pereira BAS (1988) On the flowering of Actinocladum verticillatum (Gramineae: Bambusoideae). Biotropica 20:164–166
Galindo-Leal C, Câmara IG (2003) The Atlantic forest of South America: biodiversity status, threats and outlook. CABS and Island Press, Washington, DC
Garrot RA, White PJ, White CAV (1993) Overabundance: an issue for conservation biologists? Conserv Biol 7:946–949
Gniazdowska A, Bogatec R (2005) Allelopathic interactions between plants. Multi site action of allelochemicals. Acta Physiol Plant 27:395–407
González L, Souto XC, Reigosa MJ (1995) Allelopathic effects of Acacia melanoxylon R.Br. phyllodes during their decomposition. For Ecol Manag 77:53–63
González ME, Veblen TT, Donoso C, Valeria L (2002) Tree regeneration responses in a lowland Notophagus-dominated forest after bamboo dieback in South-Central Chile. Plant Ecol 161:59–73
Goodall J, Witkowski ETF, Ammann S, Reinhardt C (2010) Doe sallelopathy explain the invasiveness of Campuloclinium macrocephalum (pompomweed) in the South African grassland biome? Biol Invasions 12:3497–3512
Griscom BW, Ashton PMS (2003) Bamboo control of forest succession: Guadua sarcocarpa in Southeastern Peru. For Ecol Manag 175:445–454
Griscom BW, Ashton PMS (2006) A self-perpetuating bamboo disturbance cycle in a neotropical forest. J Trop Ecol 22:587–597
Griscon BW, Daly DC, Ashton MS (2007) Floristics of bamboo-dominated stands in lowland terra-firma forests of southwestern Amazonia. J Torrey Bot Soc 134:108–125
Grombone-Guaratini MT, Jessen RC, Cardoso-Lopes EM, Torres LMB (2009) Allelopathic potential of Aulonemia aristulata (Doll) MacClure, a native bamboo of Atlantic Rain Florest. Allelopath J 24:183–190
Grombone-Guaratini MT, Gaspar M, Oliveira VF, Torres MAGM, Nascimento MA, Aidar MMP (2013) Atmospheric CO2 enrichment greatly increases photosynthesis and growth in a woody tropical bamboo from the Brazilian Atlantic Forest. N Z J Bot 51:275–285
Guilherme FAG, Oliveira-Filho AT, Appolinário V, Bearzoti E (2004) Effects of flooding regime and woody bamboos on tree community dynamics in a section of tropical semideciduous forest in South-Eastern Brazil. Plant Ecol 174:19–36
Inderjit (1996) Plant phenolics in allelopathy. Bot Rev 62:186–202
Inderjit (1998) Influence of Pluchea lanceolata (Asteraceae) on selected soil properties. Am J Bot 85:64–69
Inderjit, Duke SO (2003) Ecophysiological aspects of allelopathy. Planta 217:529–539
Inderjit, Nilsen ET (2003) Bioassays and field studies for allelopathy in terrestrial plants: progress and problems. Crit Rev Plant Sci 22:221–238
Inderjit, Weiner J (2001) Plant allelochemicals interference or soil chemical ecology? Perspect Plant Ecol Evol Sys 4:3–12
Inderjit, Weston LA, Duke SO (2005) Challenges, achievements and opportunities in allelopathy research. J Plant Inter 1:69–81
Inderjit, Seastedt TR, Callaway RM, Pollock JL, Kaur J (2008) Allelopathy and plant invasions: traditional, congeneric, and bio-geographical approaches. Biol Invasion 10:875–890
Inderjit, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662
Ishii-Iamamoto EL, Abrahim D, Sert MA, Bonato CM, Kelmer-Bracht AM, Bracht A (2006) Mitochondrial as a site of allelochemical action. In: Reigosa MJ, Pedrol N, González L (eds) Allelopathy: a physiological process with ecological implications. Springer, Dordrecht, pp 267–284
Kaur H, Inderjit, Kaushik S (2005) Cellular evidence of allelopathic interference of benzoic acid to mustard (Brassica juncea L.) seedling growth. Plant Physiol Biochem 43:77–81
Kaur H, Kaur R, Kaur S, Baldwin IT, Inderjit (2009) Taking ecological function seriously: soil microbial communities can obviate allelopathic effects of released metabolites. PLoS One 4:e4700. doi:10.1371/journal.pone.0004700
Larpkern P, Moe SR, Totland Ǿ (2009) The effects of environmental variables and human disturbance on woody species richness and diversity in a bamboo–deciduous forest in northeastern Thailand. Ecol Res 24:147–156
Larpkern P, Moe SR, Totland Ǿ (2011) Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 165:161–168
Lehman ME, Blum U (1999) Evaluation of ferulic acid uptake as a measurement of allelochemical dose: effective concentration. J Chem Ecol 25:2585–2600
Li FM, Hu HY (2005) Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl Environ Microbiol 71:6545–6553
Lima RAF, Rother DC, Muller AE, Lepsch I, Rodrigues RR (2012) Bamboo overabundance alters forest structure and dynamics in the Atlantic forest hotspot. Biol Conserv 147:32–39
Lorenzo P, Palomera-Pérez A, Reigosa MJ, González L (2012) Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol 212:403–412
Mathesius U (2001) Flavonoids induced in cell undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J Exp Bot 52:419–426
Montti L, Villagra M, Campanello PI, Gatti MG, Goldstein G (2013) Functional traits enhance invasiveness of bamboo over co-occurring tree saplings in the semi-deciduous Atlantic Forest. Acta Oecol 37:361–368
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Perry LG, Thelen GC, Ridenour WM, Callaway RM, Paschke MW, Vivanco JM (2007) Concentrations of the allelochemical (±)-catechin in Centaurea maculosa soils. J Chem Ecol 33:2337–2344
Preston CA, Hazel B, Baldwin IT (2002) Methyl jasmonate as an allelopathic agent: sagebrush inhibits germination of a neighboring tobacco, Nicotiana attenuata. J Chem Ecol 28:2343–2369
Rice EL (1984) Allelopathy. Academic Press, Orlando
Roessner U, Wagne C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J 23:131–142
Rother DC, Alves KJF, Pizo MA (2013) Avian assemblages in bamboo and non-bamboo habitats in a tropical rain forest. Emu 113:52. doi:10.1071/MU12017
Sánchez-Moreiras AM, Reigosa MJ (2005) Whole plant response of lettuce after root exposure to BOA (2(3H)-Benzoxazolinone. J Chem Ecol 31:2689–2703
Schultz M, Wieland I (1999) Variation in metabolism of BOA among species in various field communities—biochemical evidence for co-evolutionary process in plant communities? Chemoecology 9:133–141
Shannon-Firestone S, Firestone J (2015) Allelopathic potential of invasive species is determined by plant and soil community context. Plant Ecol 216:491–502
Shao-Lin P, Wen J, Qin-Fen G (2004) Mechanism and active variety of allelochemicals. Acta Bot Sin 46:757–766
Sosa T, Valadares C, Alías JC, Lobón NC (2010) Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 337:51–63
Souto XC, Gonzalez L, Reigosa MJ (1994) Comparative analysis of allelopathic effects produced by four forestry species during decomposition process in their soils in Galicia (NW Spain). J Chem Ecol 20:3005–3015
Tabarelli M, Peres CA, Melo FPL (2012) The “few winners and many losers” paradigm revisited: emerging prospects for tropical forest biodiversity. Biol Conserv 155:136–140
Thorpe AS, Thelen GC, Diaconu A, Callay RM (2009) Root exudate is allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–664
Tsai CS, Young CC (1993) Allelochemicals in rhizosphere soils of flowering and non flowering bamboo plants. Bot Bull Acad Sin 34:223–234
Wang J, Yue Y, Jiang H, Tang F (2012) Rapid screening for flavones C-glycosides in the leaves of different species of Bamboo and simultaneous quantitation of four marker compounds by HPLC-UV/DAD. Int J Anal Chem 2012:205101–225108
Weston LA, Mathesius U (2013) Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol 39:283–297
Wojakowska A, Piasecka A, García-López PM, Zamora-Natera F, Krajewski P, Marczak Ł, Kachlicki P, Stobiecki M (2013) Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry 92:71–86
Yamamoto T, Yokotani-Tomita K, Kosemura S, Yamada K, Hasegawa K (1999) Allelopathic substance exuded from a serious weed, germinating barnyard grass (Echinochloa crus-galli L.), roots. J Plant Growth Regul 18:65–67
Yamane A, Nishimura H, Mizutani J (1992) Allelopathy of yellow fieldcress (Rorippa sylvestris): identification and characterization of phytotoxic constituents. J Chem Ecol 18:683–691
Young CC, Chen SH, Tsai CS (1994) Phenolic compounds in soils. J Biomass Energy Soc China 13:61–67
Yuan Y, Wang B, Zhang S, Tang J, Tu C, Hu S, Yong JWH, Chen X (2013) Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J Plant Ecol 6:253–263
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Zhou YH, Yu JQ (2006) Allelochemicals and photosynthesis. In Reigosa MJ, Pedrol N, González L (eds) Allelopathy: a physiological process with ecological implications, Springer, Dordrecht, pp 127–139
Acknowledgments
This research was supported by Grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (Grant Nos. 05/51747-2 and 2010/17076-1) awarded to M. T. Grombone-Guaratini and Daniela A. Faria, respectively. The authors thank Kerstin Reifenrath (Associate Editor) and their anonymous reviewers for their very constructive comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Kerstin Reifenrath.
Rights and permissions
About this article
Cite this article
Jose, C.M., Brandão Torres, L.M., Torres, M.A.M.G. et al. Phytotoxic effects of phenolic acids from Merostachys riedeliana, a native and overabundant Brazilian bamboo. Chemoecology 26, 235–246 (2016). https://doi.org/10.1007/s00049-016-0224-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-016-0224-y