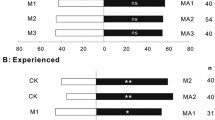
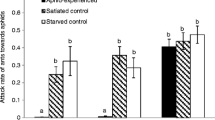
Abstract
The mutualistic relationships between certain ant and aphid species are well known, the primary benefits being protection for the aphids and carbohydrate-rich honeydew for the ants. Questions remain, however, as to the exact semiochemical factors that establish and maintain such relationships. In this study, we used a series of treatments and associated controls placed at the end of a two-way olfactometer to determine the degree of attractiveness of a complete plant–aphid–honeydew system as well as individual components of that system. Both the olfactometer branch selected by the black garden ant (Lasius niger) and the linear speed with which ants moved through the device were measured. Study results showed that ants were attracted not just to the complete plant system and the honeydew itself, but also to the microbial flora in the absence of plant or honeydew, and specifically to a bacterium from the black bean aphid (Aphis fabae) honeydew, Staphylococcus xylosus. This bacterium produces a blend of semiochemicals that attract the ant scouts. This information suggests the presence of a naturally occurring, reliable biotic cue for detection of potential aphid partners. This would have to be confirmed in natural conditions by further field experiments. Rather than being opportunistic species that coincidentally colonize a sugar-rich environment, microorganisms living in aphid honeydew may be able to alter emissions of volatile organic compounds (VOCs), thus significantly mediating partner attraction. A bacterial involvement in this mutualistic relationship could alter the manner in which these and similar relationships are viewed and evaluated. Future studies into mutualism stability and function among macroscopic partners will likely need for transition from a two-partner perspective to a multiple-partner perspective, and consider the microbial component, with the potential for one or more taxa making significant contributions to the relationship.


Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Álvarez-Pérez S, Herrera CM, de Vega C (2012) Zooming-in on floral nectar: a first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiol Ecol 80:591–602. doi:10.1111/j.1574-6941.2012.01329.x
Attygalle AB, Morgan ED (1984) Identification of trail pheromone of the ant Tetramorium caespitum L. (Hymenoptera: Myrmicinae). J Chem Ecol 10:1453–1468
Attygalle AB, Evershed RP, Morgan ED, Cammaerts MC (1983) Dufour gland secretions of workers of the ants Myrmica sulcinodis and Myrmica lobicornis, and comparison with six other species of Myrmica. Insect Biochem 13:507–512
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press
Beck HC, Hansen AM, Lauritsen FR (2004) Catabolism of leucine to branched-chain fatty acids in Staphylococcus xylosus. J Appl Microbiol 96:1185–1193. doi:10.1111/j.1365-2672.2004.02253.x
Blaiotta G, Pennacchia C, Parente E, Villani F (2003) Design and evaluation of specific PCR primers for rapid and reliable identification of Staphylococcus xylosus strains isolated from dry fermented sausages. Syst Appl Microbiol 26:601–610. doi:10.1078/072320203770865918
Bristow CM (1991) Why are so few aphids ant-tended? In: Cutler DF, Huxley C (eds) Ant/plant interactions. Oxford University Press, pp 104–119
Cammaerts MC, Evershed RP, Morgan ED (1981) Comparative study of the mandibular gland secretion of four species of Myrmica ants. J Insect Physiol 27:225–231
Davidson EW, Rosell RC, Hendrix DL (2000) Culturable bacteria associated with the whitefly, Bemisia Argentifolii (Homoptera: Aleyrodidae). Fla Entomol 83:159–171
Dixon AFG (1985) Aphid ecology. Blackie, Glasgow
Douglas AE (1998) Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria. Buchnera Annu Rev Entomol 43:17–37. doi:10.1146/annurev.ento.43.1.17
El-Ziady S, Kennedy JS (1956) Beneficial effects of the common garden ant, Lasius niger L., on the black bean aphid, Aphis fabae Scopoli. Proc R Ent Soc Lond 31:5
Febvay G, Rahbe Y, Rynkiewicz M, Guillaud J, Bonnot G (1999) Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J Exp Biol 202(Pt 19):2639–2652
Fischer CY, Lognay GC (2012) Simple and automatic closed grinding and extraction system. J Chem Educ 89:1611–1612. doi:10.1021/ed2007907
Fischer MK, Shingleton AW (2001) Host plant and ants influence the honeydew sugar composition of aphids. Funct Ecol 15:544–550
Fischer MK, Hoffmann KH, Völkl W (2001) Competition for mutualists in an ant-homopteran interaction mediated by hierarchies of ant attendance. Oikos 92:531–541
Fischer MK, Volkl W, Schopf R, Hoffmann KH (2002) Age-specific patterns in honeydew production and honeydew composition in the aphid Metopeurum fuscoviride: implications for ant-attendance. J Insect Physiol 48:319–326
Fischer MK, Volkl W, Hoffmann KH (2005) Honeydew production and honeydew sugar composition of polyphagous black bean aphid, Aphis fabae (Hemiptera : Aphididae) on various host plants and implications for ant-attendance. Eur J Entomol 102:155–160
González-Teuber M, Heil M (2010) Pseudomyrmex ants and acacia host plants join efforts to protect their mutualism from microbial threats. Plant Signal Behav 5:890–892
Grenier AM, Nardon C, Rahbé Y (1994) Observations on the micro-organisms occurring in the gut of the pea aphid Acyrthosiphon pisum. Entomol Exp Appl 70:91–96. doi:10.1111/j.1570-7458.1994.tb01762.x
Guénard B (2007) Mutualisme fourmis pucerons et guilde aphidiphage associée : le cas de la prédation furtive. Université du Québec à Montréal
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Harada H, Ishikawa H (1997) Experimental pathogenicity of Erwinia aphidicola to pea aphid, Acyrthosiphon pisum. J General Appl Microbiol 43:363–367
Haynes S et al (2003) Diversity of bacteria associated with natural aphid populations. Appl Environ Microbiol 69:7216–7223. doi:10.1128/aem.69.12.7216-7223.2003
Herrera CM, García IM, Pérez R (2008) Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89:2369–2376. doi:10.1890/08-0241.1
Herrera CM, de Vega C, Canto A, Pozo MI (2009) Yeasts in floral nectar: a quantitative survey. Ann Bot 103:1415–1423. doi:10.1093/aob/mcp026
Herrera CM, Canto A, Pozo MI, Bazaga P (2010) Inhospitable sweetness: Nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. Proc R Soc B Biol Sci 277:747–754. doi:10.1098/rspb.2009.1485
Hölldobler B, Plowes NJR, Johnson RA, Nishshanka U, Liu C, Attygalle AB (2013) Pygidial gland chemistry and potential alarm-recruitment function in column foraging, but not solitary, Nearctic Messor harvesting ants (Hymenoptera: Formicidae: Myrmicinae). J Insect Physiol 59:863–869
Hussain A, Forrest JM, Dixon AF (1974) Sugar, organic acid, phenolic acid and plant growth regulator content of extracts of honeydew of the aphid Myzus persicae and of its host plant, Raphanus sativus. Ann Appl Biol 78:65–73. doi:10.1111/j.1744-7348.1974.tb01486.x
Kirzinger MWB, Stavrinides J (2012) Host specificity determinants as a genetic continuum. Trends Microbiol 20:88–93. doi:10.1016/j.tim.2011.11.006
Kiss A (1981) Melezitose, aphids and ants. Oikos 37:382
Kloos WE (1980) Natural populations of the genus Staphylococcus. Annu Rev Microbiol 34:559–592
Leroy PD et al (2011a) Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nature Commun 2:7. doi:Artn 348
Leroy PD et al (2011) Aphid-host plant interactions: does aphid honeydew exactly reflect the host plant amino acid composition? Arthropod Plant Interact 5:193–199. doi:10.1007/s11829-011-9128-5
Liu Y, Liu Y (2002) Identification of recruitment pheromones in the harvester ant genus Pogonomyrmex. Fenxi Huaxue 30:298–300
Martineau F, Picard FJ, Ménard C, Roy PH, Ouellette M, Bergeron MG (2000) Development of a rapid PCR assay specific for Staphylococcus saprophyticus and application to direct detection from urine samples. J Clin Microbiol 38:3280–3284
Mittler TE (1958) The feeding and nutrition of large willow aphid (Tuberolachnus salignus). II. The nitrogen and sugar composition of ingested phloem sap and excreted honeydew. J Exp Biol 35:74–84
Morot-Bizot S, Talon R, Leroy-Setrin S (2003) Development of specific PCR primers for a rapid and accurate identification of Staphylococcus xylosus, a species used in food fermentation. J Microbiol Methods 55:279–286. doi:10.1016/S0167-7012(03)00159-3
Nault LR, Montgomery ME, Bowers WS (1976) Ant-aphid association: role of aphid alarm pheromone. Science 192:1349–1351
Plowes NJR, Colella T, Johnson RA, Hölldobler B (2014) Chemical communication during foraging in the harvesting ants Messor pergandei and Messor andrei. J Comp Physiol A Neuroethol Sensory Neural Behav Physiol 200:129–137
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Product Rep 24:814–842. doi:10.1039/b507392h
Stadler B (1997) The relative importance of host plants, natural enemies and ants in the evolution of life-history characters in aphids. In: Dettner K, Bauer G, Völkl W (eds) Vertical food web interactions: evolutionary patterns and driving forces. Springer, Berlin, pp 241–256. doi:citeulike-article-id:1351088
Stadler B, Dixon AFG (1999) Ant attendance in aphids: why different degrees of myrmecophily? Ecological Entomology 24:363–369
Stadler B, Dixon AFG (2005) Ecology and evolution of aphid-ant interactions. Annu Rev Ecol Evol Syst 36:345–372
Stadler B, Fiedler K, Kawecki TJ, Weisser WW (2001) Costs and benefits for Phytophagous myrmecophiles: when ants are not always available. Oikos 92:467–478
Thibout E, Guillot JF, Auger J (1993) Microorganisms are involved in the production of volatile kairomones affecting the host seeking behaviour of Diadromus pulchellus, a parasitoid of Acrolepiopsis assectella. Physiol Entomol 18:176–182
Vannette RL, Gauthier MPL, Fukami T (2013) Nectar bacteria, but not yeast, weaken a plant—pollinator mutualism. Proc R Soc B Biol Sci 280. doi:10.1098/rspb.2012.2601
Ventura M, Elli M, Reniero R, Zink R (2001) Molecular microbial analysis of Bifidobacterium isolates from different environments by the species-specific amplified ribosomal DNA restriction analysis (ARDRA). FEMS Microbiol Ecol 36:113–121. doi:10.1016/S0168-6496(01)00123-4
Verheggen FJ, Diez L, Sablon L, Fischer C, Bartram S, Haubruge E, Detrain C (2012) Aphid alarm pheromone as a cue for ants to locate aphid partners. PLoS One 7:e41841. doi:10.1371/journal.pone.0041841
Völkl W, Woodring J, Fischer M, Lorenz MW, Hoffmann KH (1999) Ant-aphid mutualisms: the impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 118:483–491
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Wiens F et al (2008) Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci USA 105:10426–10431. doi:10.1073/pnas.0801628105
Wood WF, Palmer TM, Stanton ML (2002) A comparison of volatiles in mandibular glands from three Crematogaster ant symbionts of the whistling thorn acacia. Biochem Syst Ecol 30:217–222
Wood WF, Hoang TT, McGlynn TP (2011) Volatile components from the mandibular glands of the turtle ants, Cephalotes alfaroi and Cephalotes cristatus. Biochem Syst Ecol 39:135–138
Woodring J, Wiedemann R, Volkl W, Hoffmann KH (2007) Oligosaccharide synthesis regulates gut osmolality in the ant-attended aphid Metopeurum fuscoviride but not in the unattended aphid/i Macrosiphoniella tanacetaria. J Appl Entomol 131:1–7. doi:10.1111/j.1439-0418.2006.01091.x
Acknowledgments
The authors wish to thank Dr. M. Vanderplanck, MSc H. Cawoy, Mr O. Piraux and Mr P. Hacourt for their support during this project. Christophe Fischer is financially supported by a PhD grant from the Fonds pour la formation à la Recherche dans l’Industrie et l’Agriculture (FRIA). This project is also financially supported by a Fonds de la Recherche Fondamentale Collective (F.R.F.C.–F.N.R.S.) research project (2.4600.09).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Thomas Schmitt.
Rights and permissions
About this article
Cite this article
Fischer, C.Y., Lognay, G.C., Detrain, C. et al. Bacteria may enhance species association in an ant–aphid mutualistic relationship. Chemoecology 25, 223–232 (2015). https://doi.org/10.1007/s00049-015-0188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-015-0188-3