Abstract
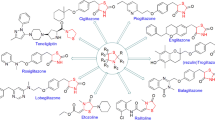
The synthesis of a novel series of substituted 5-(aminomethylene)thiazolidine-2,4-diones was achieved using a wide range of heterocyclic models derived from eight drug-like molecules. The primary aim of this study was to combine medicinally known, biologically active molecules bearing a 2° amine functionality, such as terbinafine, fluoxetine, atomoxetine, cetirizine, risperidone, aripiprazole, ziprasidone, and clopidogrel, with a thiazolidinedione ring via an amino-methylene linker. By targeting this synergistic approach to compounds with skeletal, functional, and stereochemical diversity, we have developed a simple synthetic concept to enrich the thiazolidinedione collection with various biological activities. The biological activities of the newly synthesized 5-(aminomethylene)thiazolidine-2,4-dione derivatives were explored. All compounds were found to have antibacterial activity, with compounds bearing pyridine or piperazine moieties showing good to excellent antibacterial activity. Compounds with piperazine moieties were also found to show good antifungal activity, whereas none of the synthesized compounds showed high cytotoxic activity.









Similar content being viewed by others
Notes
Dr. Reddy’s Laboratories Ltd synthesized molecules from the basic commercial raw materials.
Column purification with alumina using 2:98 methanol–dichloromethane.
Compound was dissolved in 20 mL of methanol and ethyl acetate (10:90) in a glass vial. The clear solution was filtered through a syringe equipped with sintered glass fibers. For the growth of crystals, the clear solution was sealed and a controlled vapor leak allowed for slow solvent evaporation at a constant temperature (25 °C). Growth of the compound was continued for 4–5 days. Cambridge Crystallographic Data Centre: Deposition number: CCDC 1024959; Unit cell parameters: a = 26.8266(8) Å, b = 8.4308(2) Å, c = 20.5568(5) Å, P21/c.
References
Adachi Y, Suzuki Y, Homma N, Fukazawa M, Tamura K, Nishie I, Kuromaru O (1999) The anti-ischemic effects of CP-060S during pacing-induced ischemia in anesthetized dogs. Eur J Pharmacol 367:267–273
Agrawal R, Jain P, Dikshit SN (2012) Balaglitazone: a second generation peroxisome proliferator-activated receptor (PPAR) gamma (γ) agonist. Mini-Rev Med Chem 12:87–97
Ahmad I, Beg AZ (2001) Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74:113–123
Al-Burtamani SKS, Fatope MO, Marwah RG, Onifade AK, Al-Saidi SH (2005) Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J Ethnopharmacol 96:107–112
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48(Suppl. 1):S5–S16
Bartlett PA, Entzeroth M (2006) Exploiting chemical diversity for drug discovery. Royal Society of Chemistry Biomolecular Science, RSC Publishing, Cambridge
Bhattarai BR, Kafle B, Hwang JS, Ham SW, Lee KH, Park H, Han IO, Cho H (2010) Novel thiazolidinedione derivatives with anti-obesity effects: dual action as PTP1B inhibitors and PPAR-γ activators. Bioorg Med Chem Lett 20:6758–6763
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolò F, Ottanà R, Vigorita MG (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg Med Chem 10:1077–1084
Burke MD, Schreiber SL (2004) A planning strategy for diversity-oriented synthesis. Angew Chem Int Ed 43:46–58
Burke MD, Berger EM, Schreiber SL (2003) Generating diverse skeletons of small molecules combinatorially. Science 302:613–618
Cantello BCC, Eggleston DS, Haigh D, Haltiwanger RC, Heath CM, Hindley RM, Jennings KR, Sime JT, Woroniecki SR (1994) Facile biocatalytic reduction of the carbon–carbon double bond of 5-benzylidenethiazolidine-2,4-diones. Synthesis of (±)-5-(4-{2-[methyl(2-pyridyl)amino]ethoxy}benzyl)thiazolidine-2,4-dione (BRL 49653), its (R)-(+)-enantiomer and analogues. J Chem Soc Perkin Trans 1:3319–3324
Carroll RT, Dluzen DE, Stinnett H, Awale PS, Funk MO, Geldenhuys WJ (2011) Structure-activity relationship and docking studies of thiazolidinedione-type compounds with monoamine oxidase B. Bioorg Med Chem Lett 21:4798–4803
Collins FS, Lander ES, Rogers J, Waterston RH (2004) Finishing the euchromatic sequence of the human genome. Nature 431:931–945
Culy CR, Jarvis B (2001) Repaglinide: a review of its therapeutic use in type 2 diabetes mellitus. Drugs 61:1625–1660
Day C (1999) Thiazolidinediones: a new class of antidiabetic drugs. Diabet Med 16:179–192
Diurno MV, Mazzoni O, Correale G, Monterrey IG, Calignano A, La Rana G, Bolognese A (1999) Synthesis and structure–activity relationships of 2-(substituted phenyl)-3-[3-(N, N-dimethylamino)propyl]-1,3-thiazolidin-4-ones acting as H1-histamine antagonists. Il Farmaco 54:579–583
Ergenç N, Capan G (1994) Synthesis and anticonvulsant activity of new 4-thiazolidone and 4-thiazoline derivatives. Farmaco 49:133–135
Evans AJ, Krentz AJ (1999) Recent developments and emerging therapies for type 2 diabetes mellitus. Drugs R&D 2:75–94
Grabowski K, Schneider G (2007) Properties and architecture of drugs and natural products revisited. Curr Chem Biol 1(1):115–127
Guella G, N’Diaye I, Fofana M, Mancini I (2006) Isolation synthesis and photochemical properties of almazolone, a new indole alkaloid from a red alga of Senegal. Tetrahedron 62:1165–1170
Ha YM, Park YJ, Kim JA, Park D, Park JY, Lee HJ, Lee JY, Moon HR, Chung HY (2012) Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors. Eur J Med Chem 49:245–252
Havrylyuk D, Zimenkovsky B, Lesyk R (2009) Synthesis and anticancer activity of novel nonfused bicyclic thiazolidinone derivatives. Phosphorus Sulfur Silicon Relat Elem 184:638–650
Hulin B, Clark DA, Goldstein SW, McDermott RE, Dambek PJ, Kappeler WH, Lamphere CH, Lewis DM, Rizzi JP (1992) Novel thiazolidine-2,4-diones as potent euglycemic agents. J Med Chem 35:1853–1864
Hulin B, McCarthy PA, Gibbs EM (1996) The glitazone family of antidiabetic agents. Curr Pharm Des 2:85–102
Iwatsuka H, Taketomi S, Matsuo T, Suzuoki Z (1974) Congenitally impaired hormone sensitivity of the adipose tissue of spontaneously diabetic mice, KK. Diabetologia 10:611–616
Jaeschke H (2007) Troglitazone hepatotoxicity: Are we getting closer to understanding idiosyncratic liver injury? Toxicol Sci 97:1–3
Kennedy JP, Williams L, Bridges TM, Daniels RN, Weaver D, Lindsley CW (2008) Application of combinatorial chemistry science on modern drug discovery. Comb Chem 10:345–354
Kong AP, Yamasaki A, Ozaki R, Saito H, Asami T, Ohwada S, Ko GT, Wong CK, Leung GT, Lee KF, Yeung CY, Chan JC (2011) A randomized-controlled trial to investigate the effects of rivoglitazone, a novel PPAR gamma agonist on glucose-lipid control in type 2 diabetes. Diabetes Obes Metab 13:806–813
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65:385–411
Liu H (2013) Construction of biologically potential library by diversity-oriented synthesis. Org Chem Curr Res 2:e123
Lo CP, Croxall WJ (1954) 5-Alkoxymethylenerhodanines and their reactions with rhodanines. J Am Chem Soc 76:4166–4169
Ma L, Chen J, Liang X, Xie C, Deng C, Huang L, Peng A, Wei Y, Chen L (2012) Synthesis and evaluation of 5-benzylidenethiazolidine-2,4-dione derivatives for the treatment of non-alcoholic fatty liver disease. Arch Pharm 345:517–524
Mauvais-Jarvis F, Andreelli F, Hanaire-Broutin H, Charbonnel B, Girard J (2001) Therapeutic perspectives for type 2 diabetes mellitus: molecular and clinical insights. Diabetes Metab 27(4 Pt 1):415–423
McFarland J (1907) The nephelometer: an instrument for estimating the number of bacteria in suspensions for calculating the opsonic index and vaccines. J Am Med Assoc 49:1176–1178
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 45:31–34
Mohanty S, Reddy GS, Karmakar AC (2014a) Synthesis of new 5-substituted–aminomethylene-thiazolidine-2,4-dione derivatives as potential antibacterial agents. J Appl Chem 3(1):82–90
Mohanty S, Roy AK, Kiran SP, Rafael GE, Kumar KPV, Karmakar AC (2014b) Controlling the exothermicity of O-arylation by evaporative cooling during the process development of fluoxetine hydrochloride. Org Process Res Dev 18(7):875–885
Momose Y, Meguro K, Ikeda H, Hatanaka C, Oi S, Sohda T (1991) Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chem Pharm Bull 39:1440–1445
Munos B (2009) Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discovery 8:959–968
NCCLS (2000) Method for dilution antimicrobial susceptibility test for bacteria that grow aerobically approved standards, 5th edn. National Committee for Clinical Laboratory Standards, Villanova
Pershadsingh HA, Szollosi J, Benson S, Hyun WC, Feuerstein BG, Kurtz TW (1993) Effects of ciglitazone on blood pressure and intracellular calcium metabolism. Hypertension 21:1020–1023
Pinto DGA, Saantos CMM, Silva AMS (2007) Advanced NMR techniques for structural characterization of heterocyclic structures. In: Pinho e Melo TMVD, Rocha AMdA (Eds) Recent research developments in heterocyclic chemistry, Research Signpost, Trivandrum, pp 397–475
Piscopo E, Diurno MV, Gagliardi R, Mazzoni O, Veneruso G (1989) Studies on heterocyclic compounds: 1,3-thiazolidin-4-one derivatives. IV. Biological activity of variously substituted 2,3-diaryl-1,3-thiazolidin-4-ones. Boll Soc Ital Biol Sper 65:853–859
Previtera T, Vigorita MG, Basile M, Orsini F, Benetollo F, Bombieri G (1994) 3,3′-Di [1,3-thiazolidine-4-one] system. VI. Structural and conformational studies on configurational isomers with antihistaminic activity. Eur J Med Chem 29:317–324
Rawal RK, Prabhakar YS, Katti SB, De Clercq E (2005) 2-(Aryl)-3-furan-2-ylmethyl-thiazolidin-4-ones as selective HIV-RT inhibitors. Bioorg Med Chem 13:6771–6776
Reasner CA II (1999) Promising new approaches. Diabetes Obes Metab S1:41–48
Reymond JL, van Deursen R, Blum LC, Ruddigkeit L (2010) Chemical space as a source for new drugs. Med Chem Commun 1:30–38
Scheen AJ (2001) Hepatotoxicity with thiazolidinediones: Is it a class effect? Drug Saf 24:873–888
Schimke K, Davis TM (2007) Drug evaluation: rivoglitazone, a new oral therapy for the treatment of type 2 diabetes. Curr Opin Invest Drugs 8:338–344
Schreiber SL (1998) Chemical genetics resulting from a passion for synthetic organic chemistry. Bioorg Med Chem 6:1127–1152
Schreiber SL (2000) Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287:1964–1969
Schreiber SL (2003) The small-molecule approach to biology. Chem Eng News 81:51–61
Seki H, Tokunaga T, Utsumi H, Yamaguchi K (2000) Determination of heteronuclear long-range 1H–13C and 1H–15N coupling constants of Harman by modified J-HMBC 2D NMR techniques. Tetrahedron 56:2935–2939
Sohda T, Meguro K, Kawamatsu Y (1984) Studies on antidiabetic agents. IV. Synthesis and activity of the metabolites of 5-[4-(1-methylcyclohexylmethoxy)benzyl]-2,4-thiazolidinedione (ciglitazone). Chem Pharm Bull 32:2267–2278
Sohda T, Momose Y, Meguro K, Kawamatsu Y, Sugiyama Y, Ikeda H (1990) Studies on antidiabetic agents. Synthesis and hypoglycemic activity of 5-[4-(pyridylalkoxy)benzyl]-2,4-thiazolidinediones. Arzneim-Forsch 40:37–42
Spring DR (2003) Diversity-oriented synthesis; a challenge for synthetic chemists. Org Biomol Chem 1:3867–3870
Spring DR (2005) Chemical genetics to chemical genomics: small molecules offer big insights. Chem Soc Rev 34:472–482
Strom AE, Hartwig JR (2013) One-pot anti-Markovnikov hydroamination of unactivated alkenes by hydrozirconation and amination. J Org Chem 78:8909–8914
Taxak N, Dixit VA, Bharatam PV (2012) Density functional study on the cytochrome-mediated S-oxidation: identification of crucial reactive intermediate on the metabolic path of thiazolidinediones. J Phys Chem A 116:10441–10450
Tokunaga T, Seki H, Yasuike S, Ikoma M, Kurita J, Yamaguchi K (2000) Direct detection of intramolecular Sb···N nonbonded interaction by 1H–13C and 1H–15N heteronuclear multiple bond correlation spectroscopy. Tetrahedron Lett 41:1031–1034
Walsh DP, Chang YT (2006) Chemical genetics. Chem Rev 106:2476–2530
Wu Y, Karna S, Choi CH, Tong M, Tai HH, Na DH, Jang CH, Cho H (2011) Synthesis and biological evaluation of novel thiazolidinedione analogues as 15-hydroxyprostaglandin dehydrogenase inhibitors. J Med Chem 54:5260–5264
Yoshioka T, Fujita T, Kanai T, Aizawa Y, Kurumada T, Hasegawa K, Horikoshi H (1989) Studies on hindered phenols and analogs. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. J Med Chem 32:421–428
Zidar N, Tomašić T, Šink R, Rupnik V, Kovač A, Turk S, Patin D, Blanot D, Martel CC, Dessen A, Premru MM, Zega A, Gobec S, Mašič LP, Kikelj D (2010) Discovery of novel 5-benzylidenerhodanine and 5-benzylidenethiazolidine-2,4-dione inhibitors of MurD ligase. J Med Chem 53:6584–6594
Acknowledgments
The authors thank the management of Dr. Reddy’s Laboratories Limited for giving support to carry out this work and also thank the analytical and process development team for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Reddy’s Laboratories Limited communication number: IPDO IPM-00415.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohanty, S., Reddy, S.G., RamaDevi, B. et al. An assembly of structurally diverse small and simple 5-aminomethylene derivatives of 2,4-thiazolidinedione and studies of their biological activity. Med Chem Res 24, 4037–4049 (2015). https://doi.org/10.1007/s00044-015-1447-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1447-0