Abstract
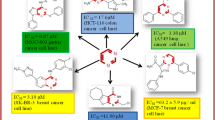
Pyridyl-benzimidazole analogues 1–11 with variable substituent on phenyl ring of phenacyl moiety were synthesized and evaluated for their urease inhibitory activity. The compounds exhibited urease inhibition with IC50 between 19.22 and 77.31 µM. Compounds 4 (IC50 = 19.22 ± 0.49 µM) showed a urease inhibition comparable to thiourea (IC50 = 21.00 ± 0.01 µM) and twofold more active than acetohydroxamic acid (IC50 = 42.00 ± 1.26 µM) (standards), respectively. Moreover, compounds 5 (IC50 = 21.55 ± 0.36 µM), 1 (IC50 = 24.37 ± 0.41 µM), 7 (IC50 = 25.44 ± 0.19 µM), 6 (IC50 = 27.62 ± 0.25 µM), 3 (IC50 = 31.32 ± 0.75 µM), 8 (40.88 ± 0.36 µM) and 9 (41.22 ± 0.42 µM) also exhibited excellent activities when compared to the standards. Compounds 2 (IC50 = 65.46 ± 0.75 µM), 10 (68.99 ± 0.33 µM) and 11 (77.31 ± 0.51 µM) showed a moderate activity. The size of the substituents and their electron donating or withdrawing affects as well as their position on phenyl apparently modulates the enzyme inhibitory activity.

Similar content being viewed by others
References
Achar KCS, Hosamani KM, Seetharamareddy HR (2010) In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur J Med Chem 45:2048–2054
Akhtar T, Khan MA, Iqbal J, Jones PG, Hameed S (2014) A facile one pot synthesis of 2-arylamino-5-aryloxylalkyl-1,3,4-oxadiazoles and their urease inhibition studies. Chem Biol Drug Des. doi:10.1111/cbdd.12297
Arfan M, Ali M, Ahmad H, Anis I, Khan A, Choudhary MI, Shah MR (2010) Urease inhibitors from Hypericum oblongifolium WALL. J Enzyme Inhib Med Chem 25:296–299
Arjmand F, Mohani B, Ahmad S (2005) Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur J Med Chem 40:1103–1110
Barker HA, Smyth RD, Weissbach H, Toohey JI, Ladd JN, Volcani BE (1960) Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5,6-dimethylbenzimidazole. J Biol Chem 235:480–488
Biron KK, Harvey RJ, Chamberlain SJ, Godd SS, Smith AA III, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW (2002) Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-Riboside with a unique mode of action. Antimicrob Agent Chemother 46:2365–2372
Boiani M, González M (2005) Imidazole and benzimidazole derivatives as chemotherapeutic agents. Mini Rev Med Chem 5:409–424
Budow S, Kozlowska M, Gorska A, Kazimierczuk Z, Eickmeier H, Colla PL, Gosselin G, Seela F (2009) Substituted benzimidazoles: antiviral activity and synthesis of nucleosides. Arkivoc 3:225–250
Cheng, X-S, Zhang J-C, You Z-L, Wang X, Li, H-H (2014) Synthesis, structures, and Helicobacter Pylori urease inhibition of hydroxamate-coordinated oxovanadium complexes with benzohydrazone ligands. Trans metal Chem. doi:10.1007/s11243-014-9802-4
Czerwonka G, Arabski M, Wasik S, Jablonska-Wawrzycka A, Rogala P, Kaca W (2014) Morphological changes in Proteus mirabilis O18 biofilm under the influence of a urease inhibitor and a homoserine lactone derivative. Arch Micro 196:169–177
Devesa SS, Blot WJ, Fraumeni JF (1998) Changing patterns in the incidence of esophageal and gastriccarcinoma in the United States. J Cancer 83:2049–2053
Elnima EI, Zubair MU, Al-Badr AA (1981) Antibacterial and antifungal activities of benzimidazole and benzoxazole. Antimicrob Agents Chemothe 19:29–32
Garuti L, Roberti M, Gentilomi G (2001) Synthesis and antiviral assays of some benzimidazole nucleosides and acyclonucleosides. II Farmaco 56:815–819
Göker H, Tunçbilek M, Ayhan G, Altanlar N (1998) Synthesis of some new benzimidazolecarboxamides and evaluation of their antimicrobial activity. II Farmaco 53:415–420
Göker H, Ozden S, Yıldız S, Boykin DW (2005) Synthesis and potent antibacterial activity against MRSA of some novel 1,2-disubstituted-1H-benzimidazole-N-alkylated-5-carboxamidines. Eur J Med Chem 40:1062–1069
Grimmett M (1996) Comprehensive heterocyclic chemistry: the structure, reactions, synthesis and uses of heterocyclic compounds, vol 3. Pergamon, Oxford, p 77
Horn J (2000) The proton-pump inhibitors: similarities and differences. Clin Ther 22:266–280
Howson CP, Hiyama T, Wynder EL (1986) The decline of gastric cancer: epidemiology of an unplanned triumph. J Epidemiol Rev 8:1–27
Iemura R, Kawashima T, Fukuda T, Ito K, Tsukamoto G (1986) Synthesis of 2-(4-substituted-1-piperazinyl)benzimidazoles as H1-antihistaminic agents. J Med Chem 29:1178–1183
Ishida T, Suzuki T, Hirashima S, Mizutani K, Yoshida A, Ando I, Ikeda S, Adachi T, Hashimoto H (2006) Benzimidazole inhibitors of hepatitis C virus NS5B polymerase: identification of 2-[(4-diarylmethoxy)phenyl]-benzimidazole. Bioorg Med Chem Lett 16:1859–1863
Kamal A, Kumar PP, Sreekanth K, Seshadri BN, Ramulu P (2008) Synthesis of new benzimidazole linked pyrrolo[2,1-c][1,4]benzodiazepine conjugates with efficient DNA-binding affinity and potent cytotoxicity. Bioorg Med Chem Lett 18:2594–2598
Kazimierczuk Z, Upcroft JA, Upcroft P, Górska A, Starooeciak B, Laudy A (2002) Synthesis, antiprotozoal and antibacterial activity of nitro- and halogeno-substituted benzimidazole derivatives. Acta Biochem Polon 49:185–195
Khan KM, Rahim F, Halim SA, Taha M, Khan M, Perveen S, Zaheer-Ul-Haq, Mesaik MA, Choudhary MI (2011a) Synthesis of novel inhibitors of β-glucuronidase based on benzothiazole skeleton and study of their binding affinity by molecular docking. Bioorg Med Chem 9:4286–4294
Khan KM, Taha M, Naz F, Khan M, Rahim F, Samreen, Perveen S, Choudhary MI (2011b) Synthesis and in vitro leishmanicidal activity of disulfide derivatives. Med Chem 7:704–710
Khan KM, Shah Z, Ahmad VU, Ambreen N, Khan M, Taha M, Rahim F, Noreen S, Perveen S, Choudhary MI, Voelter W (2012a) 6-Nitroben-zimidazole derivatives: potential phosphodiesterase inhibitors: synthesis and structure-activity relationship. Bioorg Med Chem 20:1521–1526
Khan KM, Shah Z, Ahmad VU, Khan M, Taha M, Ali S, Perveen S, Choudhary MI, Voelter W (2012b) 2,4,6-Trichlorophenylhydrazine schiff bases as DPPH radical and super oxide anion scavengers. Med Chem 8:452–461
Khan KM, Khan M, Karin A, Taha M, Ambreen N, Gojayev A, Perveen S, Choudhary MI (2013a) Xanthine oxidase inhibition by 5-aryledene N,N′-dimethylbarbituric acid derivatives. J Pak Chem Soc 35:495–498
Khan KM, Khan M, Saleem M, Taha M, Perveen S, Choudhary MI (2013b) Benzimidazoles: a new class of carbonic anhydrase inhibitors. J Pak Chem Soc 35:901–904
Khan KM, Taha M, Rahim F, Fakhri MI, Jamil W, Khan M, Rasheed S, Karim A, Perveen S, Choudhary MI (2013c) Acylhydrazide schiff bases: synthesis and antiglycation activity. J Pak Chem Soc 35:929–937
Khan KM, Naz F, Taha M, Khan A, Perveen S, Choudhary MI, Voelter W (2014a) Synthesis and in vitro urease inhibitory activity of N,N′-disubsituted thioureas. Eur J Med Chem 74:314–323
Khan KM, Jamil W, Ambreen N, Taha M, Perveen S, Morales GA (2014b) An expeditious synthetic approach towards the synthesis of bis-schiff bases (Aldazines) using ultrasound. Ultrason Sonochem 21:1200–1205
Kühler TC, Swanson M, Shcherbuchin V, Larsson H, Mellgard B, Sjöström JE (1998) Structure-activity relationship of 2-[[(2-pyridyl)methyl]thio]-1H- benzimidazoles as anti Helicobacter pylori agents in vitro and evaluation of their in vivo efficacy. J Med Chem 41:1777–1788
Kumara D, Jacobb MR, Reynoldsa MB, Sean M, Kerwin SM (2002) Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1. Bioorg Med Chem 10:3997–4004
Mansoor F, Anis I, Khan A, Marasini BP, Choudhary MI, Shah MR (2014) Urease inhibitory constituents from Daphne retusa. J Asian Nat Prod Res 16:210–215
Martelli A, Buli P, Cortecchia V (1981) Urease inhibitor therapy in infected renal stones. Eur Urol 7:291–293
Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, Schinkel AH (2005) Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dis 33:614–618
Mobley HLT, Hausinger RP (1989) Microbial ureases: significance, regulation, and molecular characterization. J Microbial Rev 53:85–108
Nguyen PT, Baldeck JD, Olsson J, Marquis RE (2005) Antimicrobial actions of benzimidazoles against oral streptococci. Oral Microbiol Immunol 20:93–100
Orjales A, Mosquera R, Labeaga L, Rodes R (1997) New 2-piperazinylbenzimidazole derivatives as 5-HT3 antagonists. Synthesis and pharmacological evaluation. J Med Chem 40:586–593
Ozden S, Atabey D, Yildiz S, Göker H (2005) Synthesis and potent antimicrobial activity of some novel methyl or ethyl 1H-benzimidazole-5-carboxylates derivatives carrying amide or amidine groups. Bioorg Med Chem 13:1587–1597
Serafin B, Borkowska G, Główczyk J, Kowalska I, Rump S (1989) Potential antihypertensive benzimidazole derivatives. Pol J Pharmacol Pharm 41:89–96
Taha M, Baharudin MS, Ismail NH, Khan KM, Jaafar FM, Samreen, Siddiqui S, Choudhary MI (2013) Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg Med Chem Lett 23:3463–3466
Taha M, Naz H, Rasheed S, Ismail NH, Rahman AA, Yousuf S, Choudhary MI (2014) Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules 19:1286–1301
Tiwari AK, Mishrab AK, Bajpai A, Mishra P, Singh S, Sinha D, Singh VK (2007) Synthesis and evaluation of novel benzimidazole derivative [Bz-Im] and its radio/biological studies. Bioorg Med Chem Lett 17:2749–2755
Veerakumari L, Munuswamy N (2000) In vitro effect of some antihelminthics on lactate dehydrogenase activity of Cotylophoron cotylophorum (Digenea: Paramphistomidae. Vet Parasitol 91:129–140
Wank SA (1998) G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am J Physiol 274:G607–G613
Weatherburn MW (1967) Phenol–hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Zhao Z, Arnaiz DO, Griedel B, Sakata S, Dallas JL, Whitlow M, Trinh L, Post J, Liang A, Morrissey MM, Shaw KJ (2000) Design, synthesis, and in vitro biological activity of benzimidazole based factor Xa inhibitors. Bioorg Med Chem Lett 10:963–966
Acknowledgments
The authors are thankful to the OPCW, The Netherlands, for their financial support for Project No. L/ICA/ICB/173681/12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saify, Z.S., Kamil, A., Akhtar, S. et al. 2-(2′-Pyridyl) benzimidazole derivatives and their urease inhibitory activity. Med Chem Res 23, 4447–4454 (2014). https://doi.org/10.1007/s00044-014-1015-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1015-z