Abstract
Exosomes offer new insight into cancer biology with both diagnostic and therapeutic implications. Because of their cell-to-cell communication, exosomes influence tumor progression, metastasis, and therapeutic efficacy. They can be isolated from blood and other bodily fluids to reveal disease processes occurring within the body, including cancerous growth. In addition to being a reservoir of cancer biomarkers, they can be re-engineered to reinstate tumor immunity. Tumor exosomes interact with various cells of the microenvironment to confer tumor-advantageous changes that are responsible for stromal activation, induction of the angiogenic switch, increased vascular permeability, and immune escape. Exosomes also contribute to metastasis by aiding in the epithelial-to-mesenchymal transition and formation of the pre-metastatic niche. Furthermore, exosomes protect tumor cells from the cytotoxic effects of chemotherapy drugs and transfer chemoresistance properties to nearby cells. Thus, exosomes are essential to many lethal elements of cancer and it is important to understand their biogenesis and role in cancer.



Similar content being viewed by others
Abbreviations
- MVE:
-
Multivesicular endosome
- ESCRT:
-
Endosomal-sorting complexes required for transport
- MDSC:
-
Myeloid-derived suppressor cells
- EMT:
-
Epithelial-to-mesenchymal transition
- HIF:
-
Hypoxia-inducible factor
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- ASC:
-
Adipose stem cell
References
Hoyert DL, Xu J (2012) Deaths: preliminary data for 2011. Natl Vital Stat Rep 61:1–52
Heron M (2012) Deaths: leading causes for 2009. Natl Vital Stat Rep 61:1–94
DeNardo DG, Coussens LM (2007) Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 9:212
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T (2000) Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 88:577–583
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17:320–329
Brentnall TA (2012) Arousal of cancer-associated stromal fibroblasts: palladin-activated fibroblasts promote tumor invasion. Cell Adh Migr 6:488–494
Li H, Fan X, Houghton J (2007) Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biohem 101:805–815
Johnson LM, Price DK, Figg WD (2013) Treatment-induced secretion of WNT16B promoted tumor growth and acquired resistance to chemotherapy: implications for potential use of inhibitors in cancer treatment. Cancer Biol Ther 14:90–91
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Sund M, Kalluri R (2009) Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev 28:177–183
Allen M, Louis Jones J (2011) Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol 223:162–176
Mueller MM, Fusenig NE (2004) Friends or foes: bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4:839–849
Joyce JA (2005) Therapeutic targeting of the tumor microenvironment. Cancer Cell 7:513–520
Rosenberg SA (2001) Progress in human tumour immunology and immunotherapy. Nature 411:380–384
Albini A, Sporn MB (2007) The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 7:139–147
Kucharzewska P, Belting M (2013) Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J Extracell Vesicles. doi:10.3402/jev.v2i0.20304
Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR (2011) Survivin is released from cancer cells via exosomes. Apoptosis 16:1–12
Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Sharma S, Rasool HI, Palanisamy V, Mathisen C, Schmidt M, Wong DT, Gimzewski JK (2010) Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano 4:1921–1926
Cocucci E, Racchetti G, Meldolesi J (2009) Shedding microvesicles: artefacts no more. Trends Cell Biol 19:43–51
Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R (2014) Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 289:3869–3875
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucl Acids Res 40:D1241–D1244
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I (2010) Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis 16:34–38
Trams EG, Lauter CJ, Salem N Jr, Heine U (1981) Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645:63–70
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
De Broe ME, Wieme RJ, Logghe GN, Roels F (1977) Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta 81:237–245
Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M (2014) Sorting it out: regulation of exosome loading. Semin Cancer Biol 28C:3–13
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126:5553–5565
Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S (2013) Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun 4:2712
Perez-Hernandez D, Gutiérrez-Vázquez C, Jorge I, López-Martín S, Ursa A, Sánchez-Madrid F, Vázquez J, Yáñez-Mó M (2013) The intercellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 288:11649–11661
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4:2980
Yu X, Harris SL, Levine AJ (2006) The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 66:4795–4801
Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A (2008) Exosome secretion, including DNA damage-induced p53-dependent secretory pathway, is severly compromised in TSAP6/Steap3-null mice. Cell Death Differ 15:1723–1733
Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD (2013) Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 288(14):10093–10099
Riches A, Campbell E, Borger E, Powis S (2014) Regulation of exosome release from mammary epithelial and breast cancer cells: a new regulatory pathway. Eur J Cancer 50:1025–1034
Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC (2004) A general role for Rab27a in secretory cells. Mol Biol Cell 15:332–344
Barral DC, Ramalho JS, Anders R, Hume AN, Knapton HJ, Tolmachova T, Collinson LM, Goulding D, Authi KS, Seabra MC (2002) Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest 110:247–257
Shin SJ, Smith JA, Rezniczek GA, Pan S, Chen R, Brentnall TA, Wiche G, Kelly KA (2013) Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer. Proc Natl Acad Sci USA 110:19414–19419
Savina A, Vidal M, Colombo MI (2002) The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 115:2505–2515
Savina A, Fader CM, Damiani MT, Colombo MI (2005) Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 6:131–143
Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM (2013) Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5:1159–1168
Tlsty TD, Hein PW (2001) Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev 11:54–59
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, von Eschenbach AC, Chung LW (1990) Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA 87:75–79
Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW (1991) Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res 51:3753–3761
Kanekura T, Chen X, Kanzaki T (2002) Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer 99:520–528
Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S (2000) Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett 157:177–184
Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L, Yan Y, Mao F, Zhao C, Shi Y, Xu W (2012) Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS One. doi:10.1371/journal.pone.0052465
Webber J, Steadman R, Mason MD, Tabi Z, Clayton A (2010) Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70:9621–9630
Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynsaston H, Mason MD, Tabi Z, Clayton A (2014) Differentiation of tumou-promoting stromal myofibroblasts by cancer exosomes. Oncogene. doi:10.1038/onc.2013.560
Atula S, Grenman R, Syrjänen S (1997) Fibroblasts can modulate the phenotype of malignant epithelial cells in vitro. Exp Cell Res 235(1):180–187
Gesierich S, Berezovskiy I, Ryschich E, Zöller M (2006) Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res 66:7083–7094
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410
Giordano FJ, Johnson RS (2001) Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev 11:35–40
Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK (2010) Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics 9:1085–1099
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA 110:7312–7317
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25:501–515
Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, Ramakrishna S (2013) Immune system: a double-edged sword in cancer. Inflamm Res 62:823–834
Kawamoto H, Minato N (2004) Myeloid cells. Int J Biochem Cell Biol 36:1374–1379
Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mécheri S (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170:3037–3045
Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1999) Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 147:599–610
Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L (2007) Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res 67:2912–2915
Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzie WE, Mobley J, Zhang HG (2009) Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 124:2621–2633
Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG (2007) Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol 178:6867–6875
Serafini P, Borrello I, Bronte V (2006) Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 16:53–65
Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, Michalek S, Grizzle W, Zhang HG (2010) Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol 176:2490–2499
Hong EH, Chang SY, Lee BR, Kim YS, Lee JM, Kang CY, Kweon MN, Ko HJ (2013) Blockade of Myd88 signaling induces antitumor effects by skewing the immunosuppressive function of myeloid-derived suppressor cells. Int J Cancer 132:2839–2848
Xiang X, Liu Y, Zhuang X, Zhang S, Michalek S, Taylor DD, Grizzle W, Zhang HG (2010) TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol 177:1606–1610
Luczyński W, Krawczuk-Rybak M, Stasiak-Barmuta A (2008) Myeloid-derived suppressor cells: the new mechanism of immunosuppression in cancer. Postepy Hig Med Dosw 62:18–22
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J (2014) Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget [Epub ahead of print]
Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z (2007) Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res 67:7458–7466
Abusamra AJ, Zhong A, Zheng X, Li M, Ichim TE, Chin JL, Min WP (2005) Tumor exosomes expressing Fas ligand mediate CD8 + T-cell apoptosis. Blood Cells Mol Dis 35:169–173
Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F, Adhikary D, Mautner J, Busson P (2009) Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cels. Blood 113:1957–1966
Yang C, Chalasani G, Ng YH, Robbins PD (2012) Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PLoS One. doi:10.1371/journal.pone.0036138
Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T (1998) B cells inhibit induction of T cell-dependent tumor immunity. Nat Med 4:627–630
Mincheva-Nilsson L, Baranov V (2014) Cancer exosomes and NKG2D receptor-ligand interactions: impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin Cancer Biol. doi:10.1016/j.semcancer.2014.02.010
Ashiru O, Boutet P, Fernández-Messina L, Agüera-González S, Skepper JN, Valés-Gómez M, Reyburn HT (2010) Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 70:481–489
Gehrmann U, Näslund TI, Hiltbrunner S, Larssen P, Gabrielsson S (2014) Harnessing the exosome-induced immune response for cancer immunotherapy. Semin Cancer Biol. doi:10.1016/j.semcancer.2014.05.003
Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V (2014) Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol. doi:10.1016/j.semcancer.2014.04.008
Fidler IJ (2002) The organ microenvironment and cancer metastasis. Differentiation 70:498–505
Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KA, Dyrskjøt L, Ørntoft TF, Larsen MR, Ostenfeld MS (2014) Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics 14:699–712
Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M, Wang SE (2014) Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep 4:5750
Kimbro KS, Simons JW (2006) Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer 13:739–749
Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza FL (2014) Hypoxia-inducible factors and RAB22 mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA [Epub ahead of print]
Irmisch A, Huelsken J (2013) Metastasis: new insights into organ-specific extravasation and metastatic niches. Exp Cell Res. doi:10.1016/j.yexcr.2013.02.012
Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, Wirtz D, Semenza GL (2011) Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA 108:16369–16374
Thuma F, Zöller M (2014) Outsmart tumor exosomes to steal the cancer initiating cell its niche. Semin Cancer Biol. doi:10.1016/j.semcancer.2014.02.011
Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, Sartor O, Abdel-Mageed AB (2014) Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells 32:983–997
Wang T, Diaz AJ, Yen Y (2014) The role of peroxiredoxin II in chemoresistance of breast cancer cells. Breast Cancer 6:73–80
Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, Tang JH (2014) Exosomes from drug-resistance breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. doi:10.1371/journal.pone.0095240
Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L (2012) Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One 7(12):e50999
Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB (2005) Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther 4(10):1595–1604
Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D’Ilio S, Lugini L, Violante N, Azzarito T, Majorani C, Brambilla D, Fais S (2014) Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. doi:10.1371/journal.pone.0088193
Gonzales PA, Zhou H, Pisitkun T, Wang NS, Star RA, Knepper MA, Yuen PS (2010) Isolation and purification of exosomes in urine. Methods Mol Biol 641:89–99
Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X (2012) Immune-related microRNAs are abundant in breast milk exosomes. Int J Bio Sci 8:118–123
Peng P, Yan Y, Keng S (2011) Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol Rep 25:749–762
Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, Witkin SS (2008) Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol 79:12–17
Rodriguez M, Silva J, López-Alfonso A, López-Muñiz MB, Peña C, Domínguez G, García JM, López-Gónzalez A, Méndez M, Provencio M, García V, Bonilla F (2014) Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes Chromosome Cancer 53:713–724
Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, Kim RY, Saria MG, Pastorino S, Kesari S, Krichevsky AM (2012) MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol 14:689–700
Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobi J, Westerberg K, Gottardo R, Tewari M, Hladik F (2014) Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 42:7290–7304
Skriner K, Adolph K, Jungblut PR, Burmester GR (2006) Association of citrullinated proteins with synovial exosomes. Arthritis Rheum 54:3809–3814
Properzi F, Logozzi M, Fais S (2013) Exosomes: the future of biomarkers in medicine. Biomark Med 7:769–778
Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH (2009) Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 10(1):42–46
Taylor DD, Gercel-Taylor C (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110(1):13–21
Properzi F, Logozzi M, Fais S (2013) Exosomes: the future of biomarkers in medicine. Biomark Med 7(5):769–778
Choi DS, Park JO, Jang SC, Yoon YJ, Jung JW, Choi DY, Kim JW, Kang JS, Park J, Hwang D, Lee KH, Park SH, Kim YK, Desiderio DM, Kim KP, Gho YS (2011) Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics 11(13):2745–2751
Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A (2009) Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer 100(10):1603–1607
Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T, Hamdy FC (2012) Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer 106(4):768–774
Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L (2011) Intracellular and extracellular microRNAs in breast cancer. Clin Chem 57(1):18–32
Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101(10):2087–2092
Natasha G, Gundogan B, Tan A, Farhatnia Y, Wu W, Rajadas J, Seifalian AM (2014) Exosomes as immunotheranostic nanoparticles. Clin Ther 36:820–829
Hao S, Liu Y, Yuan J, Zhang X, He T, Wu X, Wei Y, Sun D, Xiang J (2007) Novel exosome-targeted CD4 + T cell vaccine counteracting CD4 + 25 + regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8 + CTL response. J Immunol 179:2731–2740
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G (2008) Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 16:782–790
Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 3:10
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3:9
Pitt JM, Charrier M, Viaud S, André F, Besse B, Chaput N, Zitvogel L (2014) Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol 193:1006–1011
Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A (2010) Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics 9:1324–1338
Choi DS, Kim DK, Kim YK, Gho YS (2013) Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13(10–11):1554–1571
Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orlowski A, Vattulainen I, Ekroos K (1831) Sandvig K (2013) Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 7:1302–1309
Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M (2004) Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 380(Pt 1):161–171
He M, Crow J, Roth M, Zeng Y, Godwin AK (2014) Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip 14(19):3773–3780
Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, Gale BK (2014) A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem [Epub ahead of print]
Mizutani K, Terazawa R, Kameyama K, Kato T, Horie K, Tsuchiya T, Seike K, Ehara H, Fujita Y, Kawakami K, Ito M, Deguchi T (2014) Isolation of prostate cancer-related exosomes. Anticancer Res 34(7):3419–3423
De Broe ME, Borgers M, Wieme RJ (1975) The separation and characterization of liver plasma membrane fragments circulating in the blood of patients with cholestasis. Clin Chim Acta 59(3):369–372
Brocklehurst D, Wilde CE, Doar JW (1978) The incidence and likely origins of serum particulate alkaline phosphatase and lipoprotein-X in liver disease. Clin Chim Acta 88(3):509–515
Harding C, Stahl P (1983) Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun 113(2):650–658
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocyte in vitro: selective externalization of the receptor. Cell 33(3):967–978
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262(19):9412–9420
Harding CV, Heuser JE, Stahl PD (2013) Exosomes: looking back three decades and into the future. J Cell Biol 200(4):367–371
Johnstone RM, Matthew A, Mason AB, Teng K (1991) Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol 147:27–36
Gruenberg J (2001) The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol 2:721–730
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymophocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ (2006) Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20:1487–1495
Théry C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3:15
Gould SJ, Raposo G (2013) As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. doi:10.3402/jev.v2i0.20389
Orozco AF, Lewis DE (2010) Flow cytometric analysis of circulating microparticles in plasma. Cytometry 77:502–514
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104:3257–3266
Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ (2004) Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA 101:1297–1302
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ (1998) Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273:20121–20127
Author information
Authors and Affiliations
Corresponding author
Appendices
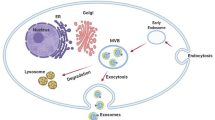
Box 1: historical perspective on exosome formation
In the 1970s, plasma membrane fragments were being isolated from human bodily fluids as well as cultured cells [27, 126, 127]. However, it was not until 1983 that two teams of researchers from the laboratories of Stahl and Johnstone independently discovered what we now term exosomes [128, 129]. The actual title of “exosome” was applied by the Johnstone group to these vesicles in 1987 [130]. Although “exosome” had previously been used by Trams et al. to describe small enzyme-containing vesicles that they observed being released from both normal and cancerous cells in culture that they postulated to have a role in communication, these vesicles do not fit the current definition of exosome [25, 131]. It was later discovered that the intracellular origin of exosomes is multivesicular endosomes (MVEs) that fuse with the plasma membrane and release their contents into the extracellular space; the connection between exosomes and MVEs lead scientists to believe that exosomes were an additional means of waste removal from cells [132, 133]. Indeed, immunological cell waste, including Major Histocompatability Complex (MHC), was found to be discarded via exosomes, but Raposo et al. [134] re-popularized the idea that exosomes were more than garbage receptacles and further postulated that exosomes could function in antigen presentation and stimulate a T cell response. Presently, exosomes are even categorized as a novel mechanism of cell-to-cell communication, particularly between tumor and stromal cells [135, 136]. For a more in-depth look at the history and nomenclature of exosomes, see [131, 137].
Box 2: use of “exosome” in scientific literature
It is important to recognize many different systems of classification of small, secreted vesicles have been used and, thus, terminology cannot necessarily be relied upon. Typically, it is agreed that exosomes are about 50–100 nm; sediment at 100,000–160,000×g or float on a sucrose gradient of 1.13–1.19 g/mL; look like a flattened sphere (transmission electron microscope, TEM), round trilobed structure with a central depression (2nN amplitude modulated—atomic force microscopy, AM–AFM), or round bulging sphere (field emission scanning electron microscope, FESEM). To ensure that research articles are indeed referring to exosomes as now defined, a few precautions should be taken:
-
careful search of the parameters used by the authors to classify the described vesicles
-
analysis of purification methods–since some are more robust than others, many false positive identifications of exosome proteins have arisen from analysis of contaminated samples [119]
-
many papers describe exosomes but incorrectly refer to them as some other type of vesicle, so it may be easy to overlook valuable exosome research
-
while some noted the presence of DNA as a difference between apoptotic vesicles and exosomes, others later concluded that exosomes also contain DNA [22, 138]
-
sometimes LAMP1 or LAMP2 is used to differentiate exosomes from other secretory vesicles; however, because of their lysosomal escape, some exosomes lack these lysosomal proteins [139–141].
For a more detailed analysis, refer to [137], which reviews the inconsistencies in nomenclature for exosomes.
Rights and permissions
About this article
Cite this article
Brinton, L.T., Sloane, H.S., Kester, M. et al. Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 72, 659–671 (2015). https://doi.org/10.1007/s00018-014-1764-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1764-3