Abstract
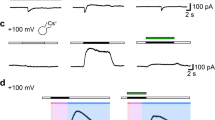
Transient receptor potential melastatin 7 (TRPM7) is a divalent-selective cation channel fused to an atypical α-kinase. TRPM7 is a key regulator of cell growth and proliferation, processes accompanied by mandatory cell volume changes. Osmolarity-induced cell volume alterations regulate TRPM7 through molecular crowding of solutes that affect channel activity, including magnesium (Mg2+), Mg-nucleotides and a further unidentified factor. Here, we assess whether chloride and related halides can act as negative feedback regulators of TRPM7. We find that chloride and bromide inhibit heterologously expressed TRPM7 in synergy with intracellular Mg2+ ([Mg2+]i) and this is facilitated through the ATP-binding site of the channel’s kinase domain. The synergistic block of TRPM7 by chloride and Mg2+ is not reversed during divalent-free or acidic conditions, indicating a change in protein conformation that leads to channel inactivation. Iodide has the strongest inhibitory effect on TRPM7 at physiological [Mg2+]i. Iodide also inhibits endogenous TRPM7-like currents as assessed in MCF-7 breast cancer cells, where upregulation of SLC5A5 sodium-iodide symporter enhances iodide uptake and inhibits cell proliferation. These results indicate that chloride could be an important factor in modulating TRPM7 during osmotic stress and implicate TRPM7 as a possible molecular mechanism contributing to the anti-proliferative characteristics of intracellular iodide accumulation in cancer cells.





Similar content being viewed by others
References
Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG (2004) Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem 279:3708–3716
Runnels LW, Yue L, Clapham DE (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291:1043–1047
Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM et al (2001) LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411:590–595
Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG (2010) TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun 1:109
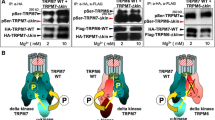
Demeuse P, Penner R, Fleig A (2006) TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol 127:421–434
Chokshi R, Matsushita M, Kozak JA (2012) Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am J Physiol Cell Physiol 302:C1004–C1011
Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114:191–200
Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD et al (2005) Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J Biol Chem 280:20793–20803
Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y et al (2012) Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Develop. Cell 22:1149–1162
Kerschbaum HH, Kozak JA, Cahalan MD (2003) Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J 84:2293–2305
Jiang J, Li M, Yue L (2005) Potentiation of TRPM7 inward currents by protons. J Gen Physiol 126:137–150
Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, Mori Y, Orser BA, Xiong ZG, Jackson MF et al (2007) TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci USA 104:16323–16328
Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 121:49–60
Runnels LW, Yue L, Clapham DE (2002) The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 4:329–336
Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A (2004) Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci USA 101:6009–6014
Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D et al (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Gen. 31:166–170
Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R et al (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Gen 31:171–174
Abed E, Moreau R (2009) Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am J Physiol Cell Physiol 297:C360–C368
Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG (2007) Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res 67:10929–10938
Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H (2009) Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol 297:C493–C502
Sahni J, Tamura R, Sweet IR, Scharenberg AM (2010) TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes. Cell Cycle 9:3565–3574
Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P (2007) Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J Immunol 179:4045–4052
Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ, So I (2008) Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci 99:2502–2509
Tani D, Monteilh-Zoller MK, Fleig A, Penner R (2007) Cell cycle-dependent regulation of store-operated I(CRAC) and Mg2+-nucleotide-regulated MagNuM (TRPM7) currents. Cell Calcium 41:249–260
Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L (2010) TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106:992–1003
Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE (2008) Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322:756–760
Wolf FI, Trapani V (2012) Magnesium and its transporters in cancer: a novel paradigm in tumour development. Clin Sci 123:417–427
Zierler S, Yao G, Zhang Z, Kuo WC, Porzgen P, Penner R, Horgen FD, Fleig A (2011) Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem 286:39328–39335
Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A key role for TRPM7 channels in anoxic neuronal death. Cell 115:863–877
Patrick L (2008) Iodine: deficiency and therapeutic considerations. Alt Med Rev 13:116–127
Arroyo-Helguera O, Anguiano B, Delgado G, Aceves C (2006) Uptake and antiproliferative effect of molecular iodine in the MCF-7 breast cancer cell line. Endo Rel Cancer 13:1147–1158
Venturi S (2001) Is there a role for iodine in breast diseases? Breast 10:379–382
Spitzweg C, Morris JC (2002) The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endo 57:559–574
Spitzweg C, Morris JC (2002) Sodium iodide symporter (NIS) and thyroid. Hormones 1:22–34
Eskin BA (1976) Dietary iodine and cancer risk. Lancet 2:807–808
Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG et al (2000) The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 6:871–878
Smerdely P, Pitsiavas V, Boyages SC (1993) Evidence that the inhibitory effects of iodide on thyroid cell proliferation are due to arrest of the cell cycle at G0G1 and G2M phases. Endocrinol 133:2881–2888
Vitale M, Di Matola T, D’Ascoli F, Salzano S, Bogazzi F, Fenzi G, Martino E, Rossi G (2000) Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinol 141:598–605
Zhang L, Sharma S, Zhu LX, Kogai T, Hershman JM, Brent GA, Dubinett SM, Huang M (2003) Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Res 63:5065–5072
Stoddard FR, Brooks AD, Eskin BA, Johannes GJ (2008) Iodine alters gene expression in the MCF7 breast cancer cell line: evidence for an anti-estrogen effect of iodine. Int J Med Sci 5:189–196
Lang F, Busch GL, Volkl H (1998) The diversity of volume regulatory mechanisms. Int J Exp Cell Physiol Biochem Pharm 8:1–45
Planells-Cases R, Jentsch TJ (2009) Chloride channelopathies. Biochim Biophys Acta 1792:173–189
Hoffmann EK, Pedersen SF (2011) Cell volume homeostatic mechanisms: effectors and signalling pathways. Acta Physiol 202:465–485
Bessac BF, Fleig A (2007) TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J Physiol (Lond) 582:1073–1086
Schomberg SL, Bauer J, Kintner DB, Su G, Flemmer A, Forbush B, Sun D (2003) Cross talk between the GABA(A) receptor and the Na-K-Cl cotransporter is mediated by intracellular Cl. J Neurophysiol 89:159–167
Lytle C, McManus T (2002) Coordinate modulation of Na-K-2Cl cotransport and K-Cl cotransport by cell volume and chloride. Am J Physiol Cell Physiol 283:C1422–C1431
Voets T, Nilius B, Hoefs S, Van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279:19–25
Thebault S, Cao G, Venselaar H, Xi Q, Bindels RJ, Hoenderop JG (2008) Role of the alpha-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP. J Biol Chem 283:19999–20007
Willhauck MJ, Sharif-Samani B, Senekowitsch-Schmidtke R, Wunderlich N, Goke B, Morris JC, Spitzweg C (2008) Functional sodium iodide symporter expression in breast cancer xenografts in vivo after systemic treatment with retinoic acid and dexamethasone. Breast Cancer Res Treat 109:263–272
Zhou J-G, Ren J-L, Qiu Q-Y, He H, Guan Y-Y (2005) Regulation of intracellular Cl- concentration through volume-regulated ClC-3 chloride channels in A10 vascular smooth muscle cells. J Biol Chem 280:7301–7308
Garcia MA, Meizel S (1999) Determination of the steady-state intracellular chloride concentration in capacitated human spermatozoa. J Androl 20:88–93
Akabas MH (2001) Chloride channels. Encyclopedia of Life Sciences 1–7
Kakazu Y, Akaike N, Komiyama S, Nabekura J (1999) Regulation of intracellular chloride by cotransporters in developing lateral superior olive neurons. J Neurosci 19:2843–2851
Arosio D, Ricci F, Marchetti L, Gualdani R, Albertazzi L, Beltram F (2010) Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat Methods 7:516–518
Collier DM, Snyder PM (2009) Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 284:29320–29325
Kusama N, Harding AM, Benson CJ (2010) Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a). J Biol Chem 285:17425–17431
Kozak JA, Kerschbaum HH, Cahalan MD (2002) Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol 120:221–235
Kozak JA, Matsushita M, Nairn AC, Cahalan MD (2005) Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J Gen Physiol 126:499–514
Lee BC, Hong SE, Lim HH, Kim do H, Park CS (2011) Alteration of the transcriptional profile of human embryonic kidney cells by transient overexpression of mouse TRPM7 channels. Int J Exp Cell Physiol 27:313–326
Chokshi R, Matsushita M, Kozak JA (2012) Sensitivity of TRPM7 channels to Mg2+ characterized in cell-free patches of Jurkat T lymphocytes. Am J Physiol Cell Physiol 302:C1642–C1651
Bachhuber T, Konig J, Voelcker T, Murle B, Schreiber R, Kunzelmann K (2005) Cl- interference with the epithelial Na+ channel ENaC. J Biol Chem 280:31587–31594
Schreiber R, Boucherot A, Murle B, Sun J, Kunzelmann K (2004) Control of epithelial ion transport by Cl- and PDZ proteins. J Memb Biol 199:85–98
Baquero Gonzales AF (2009) The regulation of epithelial sodium channels in mammalian taste receptor cells. All Grad Thes Diss Paper 418:1–201
Doyon N, Prescott SA, Castonguay A, Godin AG, Kroger H, De Koninck Y (2011) Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Comput Biol 7:e1002149
Jedlicka P, Deller T, Gutkin BS, Backus KH (2011) Activity-dependent intracellular chloride accumulation and diffusion controls GABA(A) receptor-mediated synaptic transmission. Hippocampus 21:885–898
Choi DW (1996) Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol 6:667–672
Pacheco-Alvarez D, Gamba G (2011) WNK3 is a putative chloride-sensing kinase. Int J Exp Cell Physiol Biochem Pharm 28:1123–1134
Niisato N, Eaton DC, Marunaka Y (2004) Involvement of cytosolic Cl- in osmoregulation of alpha-ENaC gene expression. Am J Physiol Renal Physiol 287:F932–F939
Ohsawa R, Miyazaki H, Niisato N, Shiozaki A, Iwasaki Y, Otsuji E, Marunaka Y (2010) Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J Cell Physiol 223:764–770
Heimlich G, Cidlowski JA (2006) Selective role of intracellular chloride in the regulation of the intrinsic but not extrinsic pathway of apoptosis in Jurkat T-cells. J Biol Chem 281:2232–2241
Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK (2010) Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol 298:C14–C25
Paravicini TM, Chubanov V, Gudermann T (2012) TRPM7: a unique channel involved in magnesium homeostasis. Int J Biochem Cell Biol 44:1381–1384
Liu XH, Chen GG, Vlantis AC, Van Hasselt CA (2009) Iodine mediated mechanisms and thyroid carcinoma. Crit Rev Clin Lab Sci 46:302–318
Rhoden KJ, Cianchetta S, Duchi S, Romeo G (2008) Fluorescence quantitation of thyrocyte iodide accumulation with the yellow fluorescent protein variant YFP-H148Q/I152L. Anal Biochem 373:239–246
Yoshida A, Taniguchi S, Hisatome I, Royaux IE, Green ED, Kohn LD, Suzuki K (2002) Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J Clin Endocrinol Metab 87:3356–3361
Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R et al (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411:595–599
Acknowledgments
Expert technical expertise provided by Stephanie Johne and Christopher Maggio. Thank you to Dr. René J.M. Bindels and Dr. Alexey Ryazanov for kindly providing pCINeo-IRES-GFP-hTRPM6 construct. This work was supported by the National Institute for General Medical Science at the National Institutes of Health [P01GM078195 to A.F.].
Conflict of interests
All authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Yu and Z. Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, H., Zhang, Z., Lis, A. et al. TRPM7 is regulated by halides through its kinase domain. Cell. Mol. Life Sci. 70, 2757–2771 (2013). https://doi.org/10.1007/s00018-013-1284-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1284-6