Abstract
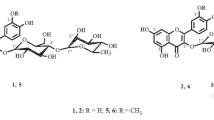
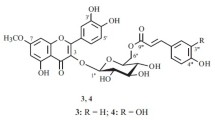
Three new derivatives of herbacetin (3,4′,5,7,8-pentahydroxyflavone) have been isolated from the rhizomes of roseroot sedum for the first time. Conclusions concerning their structures have been drawn on the basis of chemical transformations and UV, PMR, and mass spectra. The structure of herbacetin 7-O-α-rhamnopyranoside is proposed for rhodionin (I). Rhodiosin (II) has the structure of herbacetin 7-O-(3″-O-β-D-glucopyranosyl-α-L-rhamnopyranoside). The biose of which it contains a residue, which has been called rhodiose, is the first example of a 3-O-β-D-glucopyranosyl-L-rhamnopyranose residue to be found in natural flavonoid glycosides. A probable structure is put forward for the flavonolignan rhodiolin (III) — the product of the oxidative coupling of coniferyl alcohol with the 7,8-dihydroxy grouping of herbacetin.
Similar content being viewed by others
Literature cited
V. A. Kurkin, G. G. Zapesochnaya, and V. G. Klyaznika, Khim. Prir. Soedin., 581 (1982).
L. Hörhammer, H. J. Gehrmann, and L. Endres, Arch. Pharm.,262/64, 113 (1959).
T. T. Pangarova and G. G. Zapesochnaya, Khim. Prir. Soedin., 712 (1975).
G. G. Zapesochnaya and T. T. Pangarova, Khim. Prir. Soedin., 554 (1973).
G. G. Zapesochnaya, Khim. Prir. Soedin., 695 (1982).
G. G. Zapesochnaya, N. A. Tyukavkina, and S. K. Eremin, Khim. Prir. Soedin., 180 (1982).
W. Karrer, Konstitution und Vorkommen der organischen Pflanzenstoffe, Birkhaüser Verlag, Basel (1958), p. 261.
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer Verlag, Berlin (1970).
T. T. Pangarova, G. G. Zapesochnaya, and E. L. Nukhimovskii, Khim. Prir. Soedinen., 667 (1974).
G. G. Zapesochnaya, Khim. Prir. Soedin., 519 (1978).
M. Sakakibara, B. N. Timmermann, N. Nakatani, H. Waldrum, and T. J. Mabry, Phytochemistry,14, 849 (1975).
B. Janiak and R. Hänsel, Planta Med.,8, 71 (1960).
H. Wagner, in: Recent Flavonoid Research, Akademiai Kiado, Budapest, 51 (1973).
A. Pelter and R. Hänsel, Chem. Ber.,108, 790 (1975).
R. Hänsel, J. Schulz, and A. Pelter, Chem. Ber.,108, 1482 (1975).
L. Merlini, A. Zanarotti, A. Pelter, M. P. Rochefort, and R. Hänsel, J. Chem. Soc. Chem. Commun., 695 (1979).
A. Arnone, L. Merlini, and A. Zanarotti, J. Chem. Soc. Chem. Commun., 696 (1979).
C. Köppel and H. Schwartz, Org. Mass Spectrom,11, 101 (1976).
G. G. Zapesochnaya and V. A. Kurkin, Khim. Prir. Soedin., 723 (1982).
A. R. Martin, S. K. Mallick, and J. F. Caputo, J. Org. Chem.,39, 1808 (1974).
R. Hansel, J. Schulz, A. Pelter, H. Rimpler, and A. F. Rizk, Tetrahedron Lett., 4417 (1969).
K. R. Ranganathan and T. R. Seshadri, Tetrahedron Lett., 3481 (1973).
K. R. Ranganathan and T. R. Seshadri, Indian J. Chem.,12, 993 (1974).
H. Nielsen and P. Arends, Phytochemistry,17, 2040 (1978).
Additional information
All-Union Scientific-Research Institute of Medicinal Plants, Moscow. Translated from Khimiya Prirodnykh Soedinenii, No. 1, pp. 23–32, January–February, 1983.
Rights and permissions
About this article
Cite this article
Zapesochnaya, G.G., Kurkin, V.A. The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin. Chem Nat Compd 19, 21–29 (1983). https://doi.org/10.1007/BF00579955
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00579955