Summary
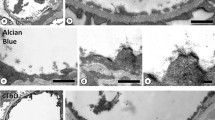
In aldehyde-fixed liver and renal cortex of rat and mouse several variations of postfixation with osmium tetroxide plus potassium ferrocyanide (FeII) were tried. Depending on the ferrocyanide concentration different staining patterns were observed in TEM.-Osmium-High Ferrocyanide [40 mM (∼1%) OsO4+36 mM (∼1.5%) FeII, pH 10.4], stains membranes and glycogen. Cytoplasmic ground substance, mitochondrial matrices and chromatin are partially extracted, cell surface coats remain unstained. Membrane contrast, but extraction too, are higher with solutions containing cacodylate- than phosphate-buffer.-Osmium-Low Ferrocyanide [40 mM (∼1%) OsO4+2 mM (∼0.08%) FeII, pH 7.4], stains cell surface coats and basal laminae, but not glycogen, except for special cases. The trilaminar structure of membranes is poorly delineated. Signs of cytoplasmic extraction are not visible. The surface coat staining is stronger and more widespread with solutions containing phosphate- instead of cacodylate-buffer; it is enhanced by section staining with lead citrate. The cell surface coat stain does not traverse tight junctions nor permeate membranes.
Similar content being viewed by others
References
Aguas AP (1982) The use of osmium tetroxide-potassium ferrocyanide as an extracellular tracer in electron microscopy. Stain Technol 57:69–73
Angermüller S, Fahimi HD (1982) Imidazole-buffered osmium tetroxide: an excellent stain for visualization of lipids in transmission electron microscopy. Histochem J 14:823–835
Bavay JC, Nicole J, Nowogrocki G, Tridot G (1968) Étude de l'acidification des solutions d'osmiates. CR Acad Sci (Paris) Sèrie C 266:1293–1295
Behrman EJ (1980a) On the oxidation state of osmium in fixed tissues. Histochem Cytochem 28:285
Behrman EJ (1980b) On the oxidation state of osmium in fixed tissues: an addendum. J Histochem Cytochem 28:1032
de Bruijn WC (1968) A modified OsO4-(double) fixation procedure which selectively contrasts glycogen. In: Bocciarelli DS (ed) 4th European Regional Conference on Electron Microscopy. Rome, vol II, pp 65–66
de Bruijn WC, den Breejen P (1975) Glycogen, its chemistry and morphological appearance in the electron microscope. II. The complex formed in the selective contrast staining of glycogen. Histochem J 7:205–229
de Bruijn WC, den Breejen P (1976) Glycogen, its chemistry and morphological appearance in the electron microscope. III. Identification of the tissue ligands involved in the glycogen contrast staining reaction with the osmium(VI)-iron(II) complex. Histochem J 8:121–142
de Bruijn WC, van Buitenen JMH (1980) X-ray microanalysis of aldehyde-fixed glycogen contrast-stained by OsVI·FeII and OsVI·RuIV complexes, J Histochem Cytochem 28:1242–1250
Dvorak AM, Hammond ME, Dvorak HF, Karnovsky MJ (1972) Loss of cell surface material from peritoneal exudate cells associated with lymphocyte-mediated inhibition of macrophage migration from capillary tubes. Lab Invest 27:561–574
Farnum CE, Wilsman NJ (1983) Pericellular matrix of growth plate chondrocytes: a study using postfixation with osmium-ferrocyanide. J Histochem Cytochem 31:765–775
Forbes MS, Sperelakis N (1980) Membrane system in skeletal muscle of the lizard Anolis carolinensis. J Ultrastruct Res 73:245–261
Forbes MS, Plantholt BA, Sperelakis N (1977) Cytochemical staining procedures selective for sarcotubular systems of muscle: modifications and applications. J Ultrastruct Res 60:306–327
Gil J, Weibel ER (1968) The role of buffers in lung fixation with glutaraldehyde and osmium tetroxide. J Ultrastruct Res 25:331–348
Glauert AM (1975) Fixation, dehydration and embedding of biological specimens. North-Holland, Amsterdam New York Oxford, p 207
Goldfischer S, Kress Y, Coltoff-Schiller B, Berman J (1981) Primary fixation in osmium-potassium ferrocyanide: The staining of glycogen, glycoproteins, elastin, an intranuclear reticular structure, and intercisternal trabeculae. J Histochem Cytochem 29:1105–1111
González-Aguilar F (1982) Cell volume preservation and the reflection coefficient in chemical fixation. J Ultrastruct Res 80:354–362
Griffith WP, Raub CJ (1980) Osmium Supplement volume 1, Gmelin Hdbch Anorgan Chemie. 8th ed. Springer, Berlin Heidelberg New York, p 347
Hammond ME, Roblin RO, Dvorak AM, Selvaggio SS, Black PH, Dvorak HF (1974) MIF-like activity in simian virus 40-transformed 3T3 fibroblast cultures. Science 185:955–957
Hayat MA (1970) Principles and techniques of electron microscopy: biological applications, Vol 1. Van Nostrand Reinhold, New York London, p 412
Havat MA (1975) Positive staining for electron microscopy. Van Nostrand Reinltold, New York London, p 361
Hayat MA (1981) Fixation for electron microscopy. Academic Press, New York London, p 501
Horobin RW (1982) Histochemistry. Gustav Fischer-Butterworth, Stuttgart New York London, p 311
Hoshino Y, Shannon WA, Seligman AM (1976) A study on ferrocyanide-reduced osmium tetroxide as a stain and cytochemical agent. Acta Histochem Cytochem 9:125–136
Hulstaert CE, Kalicharan D, Hardonk MJ (1983) Cytochemical demonstration of phosphatases in the rat liver by a ceriumbased method in combination with osmium tetroxide and potassium ferrocyanide postfixation. Histochemistry 78:71–79
Kaissling B (1980) Ultrastructural organization of the transition from the distal nephron to the collecting duct in the desert rodent Psammomys obesus. Cell Tissue Res 212:475–495
Karnovsky MJ (1971) Use of ferrocyanide-reduced osmium in electron microscopy. Proc 14th Annual Meeting Amer Soc Cell Biol, p 146
Langford LA, Coggeshall RE (1980) The use of potassium ferricyanide in neural fixation. Anat Rec 197:297–303
Larsson L (1975) Effects of different fixatives on the ultrastructure of the developing proximal tubule in the rat kidney. Ultrastruct Res 51:140–151
Latta H, Johnston WH, Stanley TM (1975) Sialoglycoproteins and filtration barriers in the glomerular capillary wall. J Ultrastruct Res 51:354–376
Lawaczeck R, Kainosho M, Chan SI (1976) The formation and annealing of structural defects in lipid bilayer vesicles. Biochim Biophys Acta 443:313–330
Lewis PR, Knight DP (1977) Staining methods for sectioned material. North Holland, Amsterdam New York Oxford, p 311
Luft JH (1971) Ruthenum red and violet. I Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec 171:347–368
Luft JH (1976) The structure and properties of the cell surface coat. Int Rev Cytol 45:291–382
Maunsbach AB (1966) The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res 15:242–282
Neiss WF (1983a) The electron density of light and dark lysosomes in the proximal convoluted tubule of the rat kidney. Histochemistry 77:63–77
Neiss WF (1983b) Extraction of osmium-containing lipids by section staining for TEM. Histochemistry 79:245–250
Payer AF, Battle CL, Peake RL (1980) Use of osmium-ferrocyanide treatment for improved lysosomal acid trimetaphosphatase reaction and subcellular detail in thyroid follicular cells. J Histochem Cyochem 28:183–186
Schiff RI, Gennaro JF (1979) The role of the buffer in the fixation of biological specimens for transmission and scanning electron microscopy. Scanning 2:135–148
Schnepf E, Hausmann K, Herth W (1982) The osmium tetroxidepotassium ferrocyanide (OsFeCN) staining technique for electron microscopy: A critical evaluation using ciliates, algac, mosses, and higher plants. Histochemistry 76:261–271
Schrével J, Gros D, Monsigny M (1981) Cytochemistry of cell glycoconjugates. Progr Histochem Cyochem 14 No 2:1–269
Staverman AJ (1952) Non-equilibrium thermodynamics of membrane processes. Trans Faraday Soc 48:176–185
Thiéry JP, Rambourg A (1974) Cytochemie des polysaccharides. J Microsc (Paris) 21:225–232
Trump BF, Eriesson JLE (1965) The effect of the fixative solution on the ultrastructure of cells and tissues. A comparative analysis with particular attention to the proximal convoluted tubule of the rat kidney. Lab Invest 14:1245–1323
Weibel ER (1979) Sterological methods, vol 1. Academic Press, New York London, p 388
White DL, Andrews SB, Faller JW, Barrnett RJ (1976) The chemical nature of osmium tetroxide fixation and staining of membranes by X-ray photoelectron spectroscopy. Biochim Biophys Acta 436:577–592
White DL, Mazurkiewicz JE, Barrnett RJ (1979) A chemical mechanism for tissue staining by osmium tetroxide-ferrocyanide mixtures. J Histochem Cytochem 27:1084–1091
White DL, Mazurkiewicz JE, Barrnett RJ (1980) On the oxidation state of osmium in fixed tissues. Auther's reply to Dr. Behrman. J Histochem Cytochem 28:285–286
Zobel CR, Beer M (1965) The use of heavy metal salts as electron stains. Int Rev Cytol 18:363–400
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft (SFB 105)
Rights and permissions
About this article
Cite this article
Neiss, W.F. Electron staining of the cell surface coat by osmium-low ferrocyanide. Histochemistry 80, 231–242 (1984). https://doi.org/10.1007/BF00495771
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00495771