Abstract
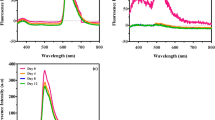
Previous results from this laboratory have shown that very low chronic doses of gamma radiation can stimulate proliferation of the Cyanobacterium Synechococcus lividus. This modification of cell proliferation occurred during the first doubling. In this paper, we have compared the metabolism of cells cultivated in a normal environment or under chronic irradiation. Incubation of the cells in a new medium induced a high superoxide dismutase (EC 1.15.1.1, SOD) activity at the 18th hour and a degradation of phycocyanin, thus demonstrating that cells were submitted to a photooxidative stress. This increase in superoxide dismutase activity was followed by concomittant peaks of glutathione reductase (EC 1.6.4.2, GR) and glucose-6-phosphate dehydrogenase (EC 1.1.1.49, G6P-DH) at the 24th hour. Irradiated cultures at a dose of 53.5 mGray/year show an earlier and higher peak of SOD, GR, and G6P-DH. In a second stage, cultures showed an earlier onset of photosynthesis under irradiation, as evidenced by an increase in pigment content and an enhancement of glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.13, GAP-DH). These results show that the radiostimulation is related to the activation of enzymes protecting against peroxides that were induced under oxidative circumstances and to the activation of a glucose catabolism via the oxidative pentose phosphate pathway.
Similar content being viewed by others
Abbreviations
- mGy:
-
milli-Gray
- SOD:
-
superoxide dismutase
- G6P-DH:
-
glucose-6-phosphate dehydrogenase
- GAP-DH:
-
glycer-aldehyde-3-phosphate dehydrogenase
- GSSG:
-
oxidized glutathione
References
Abeliovich A, Kellenberg D, Shilo M (1974) Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol 19:379–382
Burton K (1956) A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. J Biochem 62:315
Conter A, Dupouy D, Planel H (1983) Demonstration of a biological effect of natural ionizing radiations. Int J Radiat Biol 43:421–432
Conter A, Dupouy D, Planel H (1984a) Influence of growth phase on radiation stimulation of proliferation in Synechococcus lividus in culture. Rad Res 99:651–658
Conter A, Dupouy D, Planel H (1984b) Light modulation of radiosensitivity of Synechococcus lividus to very low doses of ionizing radiations. Env Exp Bot 24:229–237
Croute F, Soleilhavoup JP, Vidal S, Dupouy S, Planel H (1982) Paramecium tetraurelia growth stimulation under low-level chronic irradiation. Investigations on a possible mechanism. Rad Res 92:560–567
Cseke CS, Balogh A, Farkas GL (1981) Redox modulation of glucose 6-P-dehydrogenase in Anacystis nidulans and its “uncoupling” by phage infection. FEBS Lett 126:85–88
Dupouy D, Conter A, Croute F, Murat M, Planel H (1985) Sensitivity of Synechococcus lividus to hydrogen peroxide. Env Exp Botany 25:339–347
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Kaiser WM (1979) Reversible inhibition of the Calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta 145:377–382
Kow YW, Smyth DA, Gibbs M (1982) Oxidation of reduced pyridine nucleotide by a system using ascorbate and hydrogen peroxide from plants and algae. Plant Physiol 69:72–76
Kratz WA, Myers J (1955) Nutrition and growth of several blue-green algae. Am J Bot 42:282–287
Lau RH, Mac Kenzie MM, Doolittle WF (1977) Phycocyanin synthesis and degradation in bacterium Anacystis nidulans. J Bact 132:771–778
Lohr GW, Waller HD (1974) Glucose 6-P dehydrogenase. In: Bergmeyer (ed) Methods of enzymatic analysis, vol II. Academic Press, New York, pp 636–643
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marker AF (1972) The use of acetone and methanol in the estimation of chlorophyll in the presence of pheophytin. Fresh Water Biol 2:361–385
Myers J, Kratz AW (1955) Relation between pigment content and photosynthetic characteristics in a blue-green alga. J Gen Physiol 39:11–22
Planel H, Soleihavoup JP, Tixador R, Croute F, Richoilley G (1976) Demonstration of a stimulating effect of natural ionizing radiation and of very low radiation doses on cell mutliplication. International Atomic Energy Agency STI/PUB 409:127–140
Puget K, Michelson AM (1974) Iron containing superoxide dismutase from luminous bacteria. Biochem 56:1255–1267
Udvardy J, Balogh A, Farkas GL (1982) Modulation of glycer-aldehyde-3-phosphate-dehydnogenase in Anacystis nidulans by glutathione. Arch Microbiol 133:2–5
Udvardy J, Borbely G, Juhasz A, Farkas GL (1984) Thioredoxins and the redox modulation of glucose-6-phosphate dehydrogenase in Anabaena sp. strain PCC 7120 vegetative cells and heterocysts. J of Bacteriol 157:681–683
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Conter, A., Dupouy, D., Delteil, C. et al. Influence of very low doses of ionizing radiation on Synechococcus lividus metabolism during the initial growth phase. Arch. Microbiol. 144, 286–290 (1986). https://doi.org/10.1007/BF00410964
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00410964