Abstract
The availability of light and its efficient utilisation is a major limiting factor in large scale algal cultures. While algae predominantly use the blue and red spectral regions, a significant amount of incoming light energy remains untapped outside these ranges. Converting this unused light into usable wavelengths could enhance photosynthesis. This study aimed to identify the optimal spectral converter among Lumogen Red (LR), Rhodamine 8G (R8G), and Lumogen Yellow (LY), providing efficient light utilisation for algal cultivation. Chlamydomonas reinhardtii was cultivated in double-jacketed cylindrical photo-bioreactors (PBRs) using 30% UV-a and 5% UV-b fluorescent tubes. The R8G dye displayed a slight wavelength shift with a sharp peak at about 498 nm. The LY dye demonstrated several sharp peaks at the green and blue light spectra evident of photo-degradation. The LR dye maintained better photo-stability compared to R8G and LY. Carbohydrate, lipid and protein were produced early, whereas biomass increased after day 4, as a result of photo-acclimation. The LR dye converted and emitted 3.17 × 10-19 J photon-1, enhancing biomass production and increasing photochemical energy utilisation [Y(II)] while decreasing regulated energy dissipation [Y(NPQ)]. Pigment biosynthesis was initially increased and then reduced to counteract heightened irradiation as a means of photo-protection. LR had a 1.6- and 2.9-fold up-regulation of the RuBisCo gene expression. The dye-based system correlated with improved growth, lipid, protein, carbohydrate, and pigment production. The dye-based wavelength conversion system was interlinked to the sustainable development goals addressing environmental, economic, and social aspects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emerging microalgal biotechnological applications have established the potential for algae to mitigate some of the global challenges. For example, microalgal metabolites (e.g., proteins, carbohydrates, lipids, pigments, carotenoids, vitamins, and amino acids) can be used to provide sustainable feedstocks for various industries related to food and feed, biofuels, nutraceutical, and pharmaceutical (Sathasivam et al. 2019; Deviram et al. 2020; Kumar et al. 2022). However, there are still several drawbacks that need to be resolved. Light irradiance level (quantity) and spectral composition (quality) are critical factors influencing microalgal photosynthetic efficiency (PE) (defined as the portion of available incident light within the solar spectrum used to store chemical energy in biomass), carbon fixation capacity, cell growth, and metabolism (Ma et al. 2019; Li et al. 2021; Paul et al. 2022). Algae utilise photosynthetically active radiation (PAR) distributed within the spectral regions of 400 – 500 nm (blue) and 600 – 700 nm (red) (Nwoba et al. 2019). However large amounts of the incoming light energy, such as ultraviolet (UV) and infrared (IR), fall outside this region and are transmitted through the culture medium (Sung et al. 2018; Nwoba et al. 2019). When absorbed, microalgae growth can be negatively affected by high-energy UV photons, which can damage DNA and cause genetic defects and cellular death. Similarly, IR radiation can lead to overheating and photo-oxidation, further impairing microalgae growth.
Various approaches utilising specific PBR configurations, such as optical screening infrared, spectral selection using waveguides, wavelength filtering UV, and spectral conversion UV, have been proposed to supply specific wavelengths to the algal cultures (Vadiveloo et al. 2015; Gifuni et al. 2018); yet these have not been successfully implemented at a large scale due to the insufficient quantities of light delivered (Duarte and Costa 2018). Furthermore, technologies, such as spectral filtration, tend to reduce the transmission of wavelengths to the cultures, and consequently, the filtered wavelengths remain unused (Michael et al. 2015; Nwoba et al. 2019). The spectral conversion of light will increase the range of solar radiation used for photosynthesis (Najafpour et al. 2022).
Organic fluorescent dyes can be used as incident/UV light converters as they can concentrate, direct, and scatter light. The dyes have high quantum efficiency, wide absorption and narrow emission bands, high absorption coefficients, and show good separation between the absorption and emission bands (Khoobkar and Delavari Amrei 2020). As incident radiation passes through an organic dye it is absorbed by the luminescent species, converted, and then emitted as light with a higher potential for photosynthesis (Abou-Kana 2012; Hassan et al. 2014). The electrons produced by photosynthesis have several allocations: functional biomass (mainly proteins), storage molecules (carbohydrates and lipids), maintenance, and respiration (Sathasivam et al. 2019; Najafpour et al. 2022). Thus, dye spectral conversion can be used to trigger the production of high-value metabolites by providing specific wavelengths to algae. Consequently, using fluorescent dyes to increase usable light availability from sunlight for microalgal production would greatly reduce the need for artificial lighting systems to provide the correct wavelengths (Seo et al. 2015; Sung et al. 2018). These advantages make spectral conversion a promising technology that can be applied to microalgal cultivation systems.
Spectral conversion could potentially offer significant advantages; hence, several studies have explored this innovative approach in the context of algae (Seo et al. 2015; Vadiveloo et al. 2015; Sung et al. 2018). Khoobkar and Delavari Amrei (2020), employed Rhodamine 6G (R6G) to increase the growth in Chlorella sp. They found that the biomass productivity and the maximum specific growth rate under the R6G filtered irradiation were increased by 60 and 23%, respectively. Sung et al. (2018), used diphenylanthracene and Rhodamine 101 to improve the sunlight utilisation efficiency in Nannochloropsis gaditana. These fluorescent dyes supplemented the blue and red spectra, while increasing biomass productivity by 35 and 40%, respectively. These studies cited focussed mainly on algal growth and lipid production without thoroughly investigating the underlying mechanisms driving growth and the diverse responses related to metabolite biosynthesis. Additionally, previous research has not comprehensively analysed dye fluorescence, including aspects such as dye spectra, emission capabilities, and photo-stability. Hence, this research evaluated the potential of spectral conversion for its application to microalgal cultivation systems. The study objectives were fourfold: (1) evaluate three organic dye solutions as spectral converters of UV; (2) illustrate the effect of increased dye-mediated PAR availability on microalgal growth and physiology; (3) develop an efficient dye-based strategy to increase biomass/metabolite production in Chlamydomonas reinhardtii; (4) understand the correlation with the sustainable development goals (SDGs).
Materials and methods
Photo-bioreactor design and operation
Experiments were conducted in borosilicate glass double-jacketed cylindrical photo-bioreactors (PBRs). The inner zone included the microalgal biomass, while the outer jacket contained the dye solutions. The path length between the layers was 10 mm thick and the inner zones had a working volume of 3.5 L (dimensions: 240 mm [height], 140 mm [diameter]). The outer jackets were 300 mm high with a 170 mm diameter. Submersible pumps (Resun, SP-600, 220V-5W, a flow rate of 260 L h-1, China) were used to reduce the self-shading effect and provide mixing inside the PBRs. The PBRs were housed in an incubator equipped with 220 V electric fans to regulate air flow and prevent heating (Supplementary Fig. S1). The dyes used in experimentations were stimulated using fluorescent tubes containing 30% UV-a and 5% UV-b (Reptistar T8 [F18W/6500K]) (Sylvania, UK), (Supplementary Fig. S2). The PAR, UV-a, and UV-b radiations were measured using Vernier probes (PAR-BTA SQ-110 20772, UVA-BTA UVA1610R1, and UVA-BTA UVB3515R2, respectively;Vernier, USA).
Dye fluorescence spectra
The dyes used were chosen based on previous research (Ramanna et al. 2018). Rhodamine 8G (R8G) and Lumogen Yellow (LY) were dissolved at 10 mg L-1 while Lumogen Red (LR) was dissolved at 100 mg L-1 in methanol. The concentrations used in this study were previously optimised for spectral conversion using these dyes. However, it is essential to note that the previous study primarily focused on pigment enhancement (Ramanna et al. 2018). Irradiance levels were measured by using the PAR, UV-a, and UV-b Vernier probes (Vernier, USA) to quantitatively determine the amount of additional PAR in the PBR and determine the amount of UV irradiation attenuated by the dyes. Irradiance measurements were recorded for four hours outside and inside the PBRs (without algae) at constant height (20 cm). Beer-Lamberts Law was used to calculate the amount of light absorbed by the dyes and transmitted into the PBRs (Eq. 1):
where A and T represent the light absorbance and transmittance, respectively, both expressed as a percentage. I and I0 represent the transmitted and incidental irradiance, respectively (Eq. 2). The dye fluorescent emissions were analysed using a Cary Eclipse Fluorescence Spectrofluorometer (Agilent, South Africa) equipped with a Xenon flash lamp (15W, spectral distribution [190 – 2000 nm]). The Planck relationship was used to calculate the energy of a photon (Eq. 3):
where E represents energy in Joules (J), h represents the Plank constant which is 6.63 × 10-34 J s-1, and f is the frequency. The relationship between wavelength and frequency is represented by Eq. (4):
where f is frequency, the speed of light [C] equals 3.00 × 108 m s-1, and λ is the specific emission wavelengths in meters (m).
Strain and culture conditions
Chlamydomonas reinhardtii was isolated and identified based on molecular analyses. Briefly, DNA extraction was done using the ZR Fungal/Bacterial DNA Kit (Zymo Research) according to the manufacturer's instruction. Amplification using primers 18S-AB1 (5′GGAGGATTAGGGTCCGATTCC3′) and 18S-TW4 (5′CTTCCGTCAATTCCTTTAAG3′) that targeted the conserved regions of the 18S rRNA genes was performed according to Altschul et al. (1997). The PCR products were sequenced by Inqaba Biotechnical Industries (Pty) Ltd. (South Africa). The nucleotides sequences were obtained from the Basic Local Alignment Search Tool (BLAST), were edited and aligned using BioEdit, and a phylogenetic tree was constructed using the Maximum Likelihood method in MEGA 7 software (Tamura et al. 2011) (Supplementary Fig. S3). To establish a baseline for growth, C. reinhardtii was grown photo-heterotrophically in Tris-Acetate-Phosphate (TAP) medium (Natali and Croce 2015) in the PBRs using methanol in the jacket. The algae were grown under 16/8 h light/dark periods and received approximately 75 µmol photons m-2 s-1 irradiances. The light was supplied by T8 daylight tubes (F18W), (Tungsram, Hungary). Ampicillin (50 µg mL-1) was added to the culture medium to prevent bacterial contamination.
For the dye experiments, C. reinhardtii was initially grown for 4 days under 16/08 h light/dark periods under the Reptistar T8 fluorescent tubes. Positive controls were set up where algae received light passing through methanol (photo-heterotrophic growth), while negative controls were grown in the dark (heterotrophic growth). Microalgal growth was monitored by the optical density (OD) at 750 nm (Yarnold et al. 2016). Microalgal biomass concentrations were determined by correlating OD750 with dry cell weight (DCW). For analyses 10 mL samples were centrifuged for 10 min at 2091×g. Supernatants were discarded, pellets washed with dH2O, and re-centrifuged. The pellets were harvested and dried at 50ºC for 24 h on pre-weighed watch glasses. The DCW was quantified gravimetrically (Ramanna et al. 2018). A linear correlation between OD and DCW was calculated (Supplementary Fig. S4) and the DCW was calculated using the Eq. (5) below:
All experiments were run at 2.5 L volumes, thus allowing for a minimum of 40% remaining culture. Cultivation of C. reinhardtii was performed at low OD750 (~ 0.14) due to its fast growth rate. This approach also facilitated sufficient light transmission through cultures at later growth stages. The growth rates (µ) and biomass productivity (g L-1 day-1) were calculated as per equations below (Zhu et al. 2016):
where N1 and N2 are defined as the biomass at time 1 (t1) and time 2 (t2), respectively.
Analytical analysis
The biochemical components (lipids, proteins, and carbohydrates) were recorded based on the DCW (% w/w). Microalgal protein concentrations were estimated using a modified Bradford method (Chia et al. 2015). In brief, 10 mL samples were centrifuged for 10 min at 2.091 × g. Supernatants were discarded, pellets were washed with dH2O, and re-centrifuged. The pellets were then re-suspended in 1.5 mL 0.5M sodium hydroxide. Extraction was carried out for 2 h at 100 °C in a water bath. The samples were centrifuged and the extracted proteins were obtained by collecting the supernatant. To every millilitre of supernatant, 4 mL of Bradford reagent (0.01% Coomassie blue, 4.7% ethanol, and 8.5% phosphoric acid) was added and allowed to stand for 5 min at 20 °C. Absorbance was read at 595 nm against a reagent blank using a spectrophotometer. Total protein concentrations were obtained via a calibration curve made with bovine serum albumin (BSA) (10 – 160 µg mL-1), (Supplementary Fig. S5). For quality control, 10 µg mL-1 BSA was used as the standard. Microalgal protein concentrations were determined by using the formula:
The carbohydrate content was estimated using a modified phenol-sulphuric acid method (Chia et al. 2015). In brief, 10 mL samples were centrifuged for 10 min at 2.091 × g. Supernatants were discarded, pellets were washed with dH2O, and re-centrifuged. The pellets were then re-suspended in 1 mL of dH2O and 1 mL phenol solution (10% w/v) was added. This was vortexed and 5 mL of concentrated sulphuric acid was quickly added. The mixture was left to stand for 10 min at 20 °C. The supernatant was read at 485 nm against a reagent blank. Carbohydrate concentrations were obtained from a calibration curve of glucose (10 – 160 µg mL-1), (Supplementary Fig. S6). For quality control, 10 µg mL-1 glucose solution was used as the standard. Microalgal carbohydrate concentrations were determined by using the formula below:
To analyse lipid content, 20 mL of chloroform-methanol (2:1 v/v) was added to the biomass pellets and vortexed (Seo et al. 2015). This was heated at 100 °C for 10 min in a START D microwave digester for cell disruption (Milestone S.r.l., Italy). The mixtures were centrifuged for 10 min at 2.091 × g and the biomass pellets discarded. The supernatant was transferred to pre-weighed bijou bottles and the solvent evaporated in an oven at 60 °C for 24 h. After drying, the pre-weighed bijou bottles were cooled in a desiccator and then the weight was recorded. The total lipids were quantified gravimetrically as per Eq. (10) below:
Metabolite content (%) and productivity (g L-1 day-1) was calculated as per the equations below (Zhu et al. 2016):
where G1 and G2 are defined as the metabolite at time 0 (t0) and time 1 (t1), respectively.
Chlorophylls and carotenoids were determined as per Ramanna et al. (2018). Total carotenoid, chlorophyll b, and chlorophyll a were extracted in acetone, and absorbances were recorded at 470, 645, and 662 nm, respectively.
Photosynthetic performance of C. reinhardtii under dye-mediated irradiation
Photo-physiological analyses
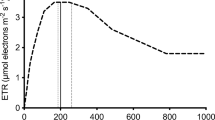
The vitality of the microalgal cellular photosynthetic apparatus was monitored using a Pulse Amplitude Modulated (PAM) fluorometer (Dual PAM-100, Heinz Walz Gmbh, Germany). Rapid light curves (RLCs) were generated by measuring the relative electron transport responses to eleven irradiances (PAR 0, 11, 18, 27, 42, 58, 100, 221, 344, 536, and 830 μmol photons m-2 s-1) for 30 s using the Saturating Pulse (SP) method (10 000 μmol photons m-2 s-1 for 1 s). Before analyses the samples were dark-adapted for 30 min. The curves yielded critical information relating to the photosynthetic energy utilisation in photosystem (PS) II, (1) effective PS2 quantum yield [Y(II)] (photochemical energy utilisation), (2) regulated energy dissipation [Y(NPQ)], and (3) non-regulated energy dissipation [Y(NO)] (Nama et al. 2019; Yadav et al. 2020). The fluorescence parameters were calculated automatically by the Dual-PAM V1.19 software based on the equations below (Heinz Walz Gmbh, Germany):
where the fluorescence yield, F, was assessed in conjunction with the application of a SP. The parameter Fm' refers to the maximum fluorescence level determined during actinic irradiation, Fm and F0 are the maximum and minimum fluorescence after dark-adaptation, respectively. qL represents the coefficient of photochemical quenching (Almeida et al. 2017; Esquivel et al. 2017). The difference between Fm' and Fm represents the dissipation of energy due to photo-regulation.
Gene expression studies
RNA extraction, cDNA synthesis, and PCR
Total RNA was extracted with the Qiagen RNeasy Plant Mini Kit (Qiagen, Germany) following the manufacturer’s protocols (Nordin et al. 2020). Total RNA concentrations, integrity, and purity were measured at 260/280 nm with an Implen NanoPhotometer (Implen, USA). Reverse transcription (RT) was carried out using the RevertAid First Strand cDNA Synthesis Kit as per the manufacturer’s protocols (Thermo Fisher Scientific, USA). The GAPDH mouse RNA template supplied with the kit was run as a positive control. The synthesised single-stranded cDNA (50 ng) was used as a substrate for the initial polymerase chain reaction (PCR). The oligonucleotide sequences of primers used are described in Supplementary Table S1. Primers were selected to target the key functional gene involved in photosynthesis (RuBisCo large subunit [rbcL]) (Giridhar Babu et al. 2017). For optimisation verification, the amplicons generated were subjected to electrophoresis on lithium acetate borate gel (Brody et al. 2004), (Supplementary Fig. S7a and b).
Real-Time qPCR
The SYBR Green qPCR assay (Thermo Fischer Scientific) was performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, USA). Reactions were performed as per the manufacturer’s protocols (Giridhar Babu et al. 2017). The 2-ΔΔCT method was used to analyse the relative gene expression (Livak and Schmittgen 2001). The 18S rRNA gene was used as the reference gene in this study, and for quality assurance, control GAPDH cDNA, no template controls (NTC), and no RT (NRT) controls were run as positive and negative controls, respectively. The control GADPH, rbcL, and 18S rRNA amplicons melted at 88, 76, and 82 ºC, respectively (Supplementary Fig. S8a and b). The CFX Manager Software version 3 for Windows was used to analyse the qPCR data and draw melt curves (Bio-Rad Laboratories). The data were plotted to represent the fold change (increase or decrease) of the RuBisCo gene in dye-irradiated cultures relative to the control sample. Gene expression studies were conducted on days 4 and 8.
Statistical analysis
Reactors were run in batch mode under the same conditions for comparison on the treatments (Supplementary Fig. S9), and all analysis from the reactors were conducted in triplicate. One sided student t-tests were used to compare and locate the significant differences in the individual means between dye-mediated and control cultures, using a 95% confidence limit (p=0.05). All statistics and graphs were analysed and prepared using GraphPad Prism (Version 9.00 for Windows, www.graphpad.com).
Results
Dye fluorescence spectra
Table 1 presents data on the UV-a and b irradiances, PAR quantities, and dye fluorescence intensities transmitted into the microalgal culture. The surface of the PBR walls received approximately 500 mW m-2 of UV-a radiation via the fluorescent tubes. The amount of UV-a transmitted inside the PBRs under the control, LR, LY, and R8G conditions represented 74, 35, 43, and 41% of the total UV-a received, respectively (Table 1). The PBRs were exposed to UV-b radiation of approximately 350 mW m-2, and 9.7, 4.9, 7.1, and 4.8% of UV-b were transmitted into the control, LR, LY, and R8G algal culture media, respectively. The transmittance measurements indicated that the dyes used in the experiment altered the spectra of light inside the PBRs. Due to their broad spectral excitation, the dyes attenuated 55 – 77% of UV-b (Table 1), which were transmitted into the PAR portion.
The PBRs surface level received 110 µmol photons m-2 s-1 of PAR via the fluorescent tubes. The quantities of PAR transmitted into the microalgal culture varied significantly among the different dyes. Under the control, LR, LY, and R8G conditions, the quantities of PAR transmitted into the culture were 72, 29, 84, and 91 µmol photons m-2 s-1, respectively (Table 1). The control allowed the passage of visible while the dyes absorbed or reflected the light. Lumogen yellow and R8G emitted the highest quantities of PAR, subsequently, these were ~ 16 and 26% higher than the control. To reduce the variability within the data, further research should improve experiments technically. The photoemission spectrum of the LR condition showed a major peak intensity between 620 and 660 nm, corresponding to the yellow-to-red region of PAR (Fig. 1a), providing effective light energy for microalgal growth (calculated as 3.17 × 10-19 J photon-1).
The distribution of photoemission spectra associated with LY dye showed several sharp peaks at the blue to green (400 – 525 nm) light spectra, (Fig. 1b). The fluorescence intensity also extended to 600 nm, representing the green to yellow PAR portion. The theoretical energy converted and released from LY was 3.99 × 10-19 J photon-1, demonstrating the ability of the dye to convert and concentrate energy in specific wavebands. However, the fluorescence intensity of the emission spectrum drastically reduced after day 0, suggesting the colour change due to photolytic decomposition and degradation of the fluorescence molecules (photo-bleaching phenomenon). The change from a yellow colour to a transparent one under prolonged irradiation was observed. The R8G dye condition showed a wavelength distribution mostly between 480 and 550 nm, representing the blue to the green PAR region (Fig. 1c). Furthermore, the energy converted and released by R8G was 3.97 × 10-19 J photon-1, suggesting that this dye could stimulate pigment and/or lipid production in algae.
Effects of dye-mediated irradiation on the growth of C. reinhardtii
Fig. 2 shows the growth profile of C. reinhardtii under dye-mediated irradiations. On day 3 the biomass concentrations reached 0.8, 1.1, 1.2, and 1.3 g L-1 for the control, LR, LY, and R8G, respectively. Dye irradiated cultures exhibited faster growth rates when compared to the control. These were 0.60, 0.67, 0.70, and 0.73, respectively. The microalgal growth dipped on day 4, but gradually increased after day 5, showing a 12-day growth period. This arrest in growth could be due to excess light irradiances provided by the dyes, not present in the control. The highest biomass concentrations were noted on day 10 which were 1.7, 1.4, 1.4, and 1.3 g L-1 for the control LR, LY, and R8G irradiated cultures, respectively (p<0.05). Growth decline after day 10 could be attributed to nutrient deprivation due to batch cultivation. The culture pH values ranged from 7 to 8, while temperatures fluctuated between 23 and 26°C throughout the study (Supplementary Fig. S10a and b).
Effects of dye-mediated irradiation on photosynthetic energy utilisation
The Y(II) values initially decreased until day 4 and then increased until day 12 (Fig. 3a). For instance, the Y(II) values increased from 0.04 to 0.10 for the control and from 0.08 to 0.13 for the LR-grown cultures. The LR grown culture had the highest Y(II) value among the dye-mediated irradiations. Similar to the Y(II) profile, the regulated dissipation Y(NPQ), increased during the second growth phase by about 31 – 77% (Fig. 3b). The Y(NO) values slightly decreased after day 4 (Fig. 3c).
Physiological parameters of C. reinhardtii grown under control (Cont.), Lumogen Red (LR), Lumogen Yellow (LY), and Rhodamine 8G (R8G) using Reptistar T8 UV-a and UV-b fluorescent tubes, (a) photochemical energy utilisation [Y(II)], (b) regulated energy dissipation [Y(NPQ)], and (c) non-regulated energy dissipation [Y(NO)]. Data are expressed as mean ± SEM (n = 3)
Effects of dye-mediated irradiation on photosynthetic gene expression
Fig. 4 shows the relative gene expression (rbcL) of C. reinhardtii grown under different UV-mediated dye irradiation. There was an increased/adjusted RuBisCO activity on day 8. An increased RuBisCO activity was observed for LR cultures when compared with both LY and R8G grown cultures. The control group did not exhibit much change in the rbcL gene expression.
Effects of dye-mediated irradiation on metabolite production
Initially, C. reinhardtii exhibited an increase in the chlorophyll productivity, (Fig. 5a). For instance, the chlorophyll productivities were 93, 108, 69, and 85 g L-1 day-1 for control, LR, LY, and R8G, respectively (day 4). The LR dye-irradiated cultures exhibited 13% higher chlorophyll content than the control. Similar to the chlorophyll pattern, the highest carotenoid contents were noticed initially followed by a declined profile along with the cultivation time (Fig. 5b). The carotenoid productivities during the initial period were 40, 90, 87, and 91 g L-1 day-1 for the control, LR, LY, and R8G grown cultures, respectively (day 4). As the study progressed, the LR profile exhibited the lowest carotenoid content compared with those grown under LY and R8G (p<0.05).
Effects of dye-mediated irradiation on biochemical components
On day 4, the initial protein productivity was 92, 189, 177, and 201 g L-1 day-1 under control, LR, LY, and R8G irradiations, respectively (Fig. 6a). This represented a 51, 48, and 54% increase in protein productivity under LR, LY, and R8G when compared to the control (p<0.05). The protein declined to 90, 112, 162, and 122 g L-1 day-1 for the control, LR, LY, and R8G irradiated cultures, respectively (day 8). However, this represented a 19, 43, and 26 % increase in protein productivity under LR, LY, and R8G when compared to the control (p<0.05).
On day 4, the initial carbohydrate productivity was 88, 156, 98, and 110 g L-1 day-1 for the control, LR, LY, and R8G irradiated cultures, respectively (Fig. 6b). The dye-irradiated cultures exhibited between 10 – 43% higher productivity when compared to the control, (p<0.05). On day 8, the carbohydrate was 86, 79, 108, and 68 g L-1 day-1 for the control, LR, LY, and R8G irradiated cultures, respectively.
The initial lipid productivity reached 64, 82, 65, and 71 g L-1 day-1 for the control, LR, LY, and R8G irradiations, respectively (day 4), (p<0.05), (Fig. 6c). This related to 21, 1, and 9% significant increase in lipid under LR, LY, and R8G irradiations, respectively. On day 8 the lipid content reached 83, 59, 84, and 66 g L-1 day-1 for the control, LR, LY, and R8G irradiations, respectively (p<0.05).
Discussion
Dye fluorescence spectra
The data showed that the amount of UV-a transmitted inside the PBRs varied under different conditions (Table 1). It has been reported that the UV-a (315 – 400 nm) spectrum could shift to the PAR (400 – 700 nm) wavelength using a spectral converting solid resin for enhancing microalgae growth rate (Delavari Amrei et al. 2014). However, it is crucial to maintain low levels of UV-a to avoid undesirable effects on algal growth and photosynthesis (Najafpour et al. 2022). Microalgae develop protective mechanisms, such as cell wall thickening and the synthesis of compatible solutes and carotenoids to respond to the harmful effects of UV-b radiation (280 – 315 nm), (Singh et al. 2019). The dyes used in the experiment altered the spectra of light inside the PBRs. The amount of UV light that would otherwise be received by the algae was reduced by ~ 50%. This was evident by the increased UV light under the control condition, and the increased PAR under dye fluorescence (Table 1). The findings showed that the dyes used in the experiment altered the quantity and quality of light transmitted into the algal culture.
LR was optimal for promoting photosynthesis in microalgae by providing light in the wavelengths that are most efficiently absorbed by the photosynthetic pigments (Fig. 1a). The energy level of red photons is approximately comparable to that of PSs in chlorophyll, promoting the photosynthetic energy demand (Seo et al. 2015), while the green light has high energy to penetrate deeper in dense algal cultures compared to red dye-irradiation (Mattos et al. 2015). It has been shown that the chlorophyll (a + b) strongly absorb red light due to the non-dense microalgae culture under investigation, where the cells closer to the illuminated surface would completely absorb PAR. The red wavelengths (600 – 700 nm) are strongly absorbed by chlorophyll-a (at 660 nm) and chlorophyll-b (at 641.8 nm). Additionally, red-light is desirable as it provides high penetration capacity to either well-mixed or non-dense microalgae cultures due to its high wavelength band and low light energy level in the PAR range (Nwoba et al. 2021).
The reduction in LY dye fluorescence intensity (Fig. 1b) indicated that it is not suitable as a spectral converter for the long-term lighting application in microalgae cultivation systems. Fluorescence intensity reduction could potentially affect the photosynthetic efficiency of the algae, but it could also increase the fraction of absorbed light energy for photosynthesis (Meagher et al. 2021). Therefore, it is important to consider the dye's photo-stability and its impact on algal photosynthetic efficiency before using it as a light converter in PBRs. The R8G dye emissions can be seen in Fig. 1c. The emissions from this dye could be beneficial for algal growth and lipid production. Previous findings reported that targeting the blue region of PAR maintained the most suitable light to biomass conversion and higher lipid content in Nannochloropsis (Vadiveloo et al. 2015). Generally, carotenoids absorb the violet/blue/green portions of PAR, and they transmit the harvested photon energy to chlorophyll a (Nwoba et al. 2019). The variation in results observed was attributed to the functionality of each dye in shifting the wavelengths present in the spectrum, converting UV light to blue, green, or red light. However, an un-optimised PAR intensity (either too low or excessive) can lead to photo-inhibition, altering the photosynthetic functionality (Nwoba et al. 2021; Ezequiel et al. 2022). Therefore, a careful selection of light wavelength and intensity should be considered for optimal algae growth and biomass production.
The sustainability of the dye technology at an industrial scale is influenced by several factors, particularly the choice of solvent and the efficiency of spectral conversion. The use of methanol as a solvent may be preferred due to its wide availability (as denatured alcohol), or its lower cost as opposed to acetone (Zehentbauer et al. 2014; Maillard et al. 2020). The efficiency of spectral conversion is highly dependent on the chemical structure of the dye and the interaction between the dye molecule and the solvent (Zehentbauer et al. 2014). Methanol is favoured as it does not absorb light in the same region as the dye. The solvent absorbs light with a molar absorption coefficient in the 700 – 900 nm range (Maillard et al. 2020). It demonstrates good solubility, optical transparency, and proper absorption and emission of the dissolvent dye (Maillard et al. 2020), in accordance with previous research in the field (Abou-Kana 2012; Zehentbauer et al. 2014). Nonetheless, to enhance the sustainability of the dye technology, considering a less expensive solvent can be a strategic approach. One such alternative could be ethanol. Ethanol is readily biodegradable compared to other solvents, thereby facilitating easier waste management. It is generally considered safer to handle than methanol. Replacing methanol with ethanol as a solvent may provide a more sustainable approach as ethanol can be derived from renewable sources like sugarcane or corn. This would further improve the ecological footprint of the dye technology.
Effects of dye-mediated irradiation on the growth of C. reinhardtii
The control cultures showed slow growth, in the early stages (< day 4), which was attributed to the UV irradiation (evident by Fig. 2). However, lower UV-mediated PFD produced more biomass toward the end of the study when compared to dye-irradiated cells (Fig. 2). The biomass productivity under dyes irradiance was between 27 – 39% higher than the control (on day 3). During the exponential phase of growth, algae increase their cellular division to reduce photosynthetic complexes in response to excess irradiation. This strategy is used to mitigate photoinhibition of the cells (Nowicka 2020; Meagher et al. 2021). The control cultures required 7 days to reach similar biomass yields as the dye-irradiated cultures (1.20 – 1.24 g L-1), demonstrating the beneficial effect of increased light availability provided by the dyes. By the 5th day there was an inhibition in cell division under dye-irradiations to match the irradiance level resulting in slower growth implying that dye-irradiations were beneficial in the rapid growth phase (day 3) but were in excess by the 5th day. Despite the transient arrest in growth on day 5, LR and R8G grown algae showed a significant difference in biomass from the control and LY irradiated cultures (p<0.05). Increases in algal growth under LY irradiation may be due to the filtering of the UV light by the LY intermediates. Transformed dye intermediates have higher absorption coefficients and thus, absorb irradiation at the same wavelength of the dye (Delgado-Sanchez 2019). After the 5th day, the growth concentrations increased by 89, 12, 54, and 18%, respectively, reaching their maximum values on day 10. From the growth and PAM fluorometry data, it was evident that the cultures were not in a steady state and were continuously acclimating to (a) the change in irradiance to light colour and (b) the decline in nutrients over time. This might account for the rather erratic growth curves shown in Fig. 2.
Effects of dye-mediated irradiation on photosynthetic energy utilisation
The Y(II) values reflect the portion of light energy that was utilised in PSII (Fig. 3a). In this study, the initial decrease and then increase in Y(II) values suggested that the microalgae were adapting to the new cultivation conditions during the initial phase. The LR culture exhibiting a better energy utilisation efficiency, consistent with the higher biomass concentration observed in the LR group (as Fig. 2). This finding implied that the algae repaired the photo-damage caused by UV light (Fig. 3c, discussed below), leading to an increase in the remaining portion of energy available for biomass growth or be further dissipated as either heat or Chl a fluorescence, detailed in Ezequiel et al. (2022) and Herdean et al. (2023).
The increase in the Y(NPQ) indicated that the algal biomass executed essential regulatory mechanisms to protect its photosynthetic apparatus from damage due to excess photon absorption (Fig. 3b). Microalgae can induce photo-protective mechanisms when absorbing energy in surplus amounts to the capacity of downstream metabolic reactions. The increase in Y(NPQ) suggested that the algae initiated a photo-acclimation period, where the photosynthetic apparatus was adjusted according to changes in light irradiance. This adjustment in the algae photochemistry was due to exposure to either higher or lower photon flux densities, as noted by Meagher et al. (2021).
The findings of the Y(NO) analysis indicated that during the initial growth phase, algal biomass suffered severe damage caused by light (Fig. 3c). This was reflected in the higher Y(NO) observed for this phase. The increased Y(NO) is presumed due to the deficiency of both the photochemical energy conversion and the protective regulatory mechanisms to maintain excitation energy, suggesting that the algae were not yet acclimated to the light conditions (Nowicka 2020; Meagher et al. 2021). However, as the experiment progressed, Y(NO) gradually decreased (> day 4), signifying that the fraction of unregulated energy dissipation played a role during photo-acclimation (Yadav et al. 2020). A higher Y(NO) was observed for LY and R8G than LR demonstrating that the majority of light energy was dissipated through the unregulated pathway rather than being directed to fuel the photochemical processes. The increase in Y(II) and Y(NPQ) and the stabilisation of Y(NO) after day 6 could also be ascribed to photo-acclimation, further indicating that C. reinhardtii retained the physiological means to utilise and regulate energy. This could also explain the increase in algal growth at that point. As mentioned, cultures grown under increased dye-mediated PFDs demonstrated active growth and division to reduce light penetration through the medium, thereby attempting to reduce photo-inhibition (Nowicka 2020; Meagher et al. 2021). The PAM findings also implied that the initial 4-day growth under UV light may not have been a sufficient acclimation period. Too much light energy was given off as Y(NO) throughout the study, subsequently, a longer acclimation was required. Vadiveloo et al. (2015) acclimated Nannochloropsis sp. for 20 days to a new wavelength before experiments and the experiments were carried out in a semi-continuous culture mode so that the algae remained in a steady-state with respect to nutrients. In the current study the cultures were still acclimating while they were growing, hence improved results would have been obtained if they were pre-acclimated to the different spectra (and irradiances) for the treatments.
Effects of dye-mediated irradiation on photosynthetic gene expression
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is a carboxylating enzyme used to fix CO2, representing the entry point of carbon into the Calvin–Benson–Bassham cycle. The data in Fig. 4 suggested that the adaptation of the photosynthetic apparatus composition to the irradiance intensity led to an increase in the RuBisCO activity. Microalgae utilised the photons of the PAR-portion and acclimated to the light condition, promoting the relative gene expression. This was verified by the increased rbcL gene expression observed for all the dye-mediated irradiations, with the highest expression observed in the LR group. The increased RuBisCO activity in the LR group was attributed to the better energy utilisation efficiency observed in this group, which resulted in a higher biomass concentration compared with both LY and R8G grown cultures. The rbcL gene expression for the control group was constant during the photo-acclimation phase, which suggested a stressful condition where the microalgae had to allocate resources to repair damage caused by excess photons. Overall, the data in Fig. 4 implied that dye-grown microalgae adapted to the irradiance condition by upregulating the RuBisCo gene expression to fulfil the energy generation needed to promote shorter growth and cell cycles (Sanchez-Tarre and Kiparissides 2021).
Effects of dye-mediated irradiation on metabolite production
Dye solutions used in this study facilitated the absorption and transfer of light energy via photosynthesis, leading to higher chlorophyll and carotenoid productivity in the dye-irradiated cultures compared to the control (Fig. 5a and b). The higher chlorophyll content in the dye-irradiated cultures implied that the dyes facilitated the absorption and transfer of light energy via photosynthesis. These findings supported previous research that demonstrated the enhancement of photosynthetic pigments including chlorophyll when microalgae were exposed to optimal light conditions (Nawrocki et al. 2020; Nowicka 2020).
The initial increase in carotenoid productivity in all cultures reflected the stress condition. Apart from capturing light, carotenoids also play a role in protecting the photosynthetic apparatus from photo-oxidative damage (NPQ) (Nawrocki et al. 2020; Nowicka 2020). The lower carotenoid productivity in the LR culture implied that C. reinhardtii grown under LR was subjected to lower light stress compared to those grown under LY and R8G (Fig. 5b).
Effects of dye-mediated irradiation on biochemical components
The increase in protein productivity (Fig. 6a), was attributed to the potential oxidative stress induced by excess irradiances, leading to alterations in protein-pigment complexes that promote microalgal growth (Gifuni et al. 2018). This was validated by the concomitant decrease in Y(II) and increases in Y(NPQ) and Y(NO), resulting from excess irradiances (Fig. 3a, b, and c, respectively). The increase in protein content in cultures grown under LY can be explained by the enhanced photosynthetic activity resulting from increased chlorophyll content and biomass. The photolytic decomposition and degradation of the fluorescence molecules changed the light environment for the algae over time (Fig. 1b). This influenced the data which makes the results difficult to interpret. The increase in protein production during photo-acclimation can serve as an indicator of the adaptation of microalgae to the growth conditions, further supporting the findings of increased chlorophyll and biomass observed in the study (Fig. 5a and Fig. 2, respectively).
The results showed that increased dye irradiance significantly affected the metabolic use of energy, as evidenced by the increased Y(NPQ) values in Fig. 3b. Carbohydrates not only have functions relevant to algal photosynthesis but also act as free radical scavengers and structural components regulating turgor and permitting multicellularity (Chia et al. 2015). The decreased carbohydrate productivity (Fig. 6b), during later stages (> day 4) could be influenced by the rate of reduced carbon production and consumption in photosynthesis and growth, as reported in previous studies (Sung et al. 2018; Vuppaladadiyam et al. 2018). This may account for the increases in growth after day 6. The findings showed that dye-mediated cultures had a higher capacity to produce carbohydrates than the control cultures, indicating their potential for biotechnological applications.
The initial lipid productivity in microalgae increased under all dye-mediated cultures when compared to the control (Fig. 6c). This was attributed to the triggered physiological synchronisation that maintained cellular redox homeostasis in response to UV irradiance (day 4), (Chia et al. 2015). However, during later stages (> day 4), growth increased at the expense of lipid production. The degradation of lipids may have been necessary to provide adequate energy required for active growth and cellular division. Apart from being structural components in the PSs serving as electron sinks of excess energy, lipids provide sources of energy for growth (Sung et al. 2018; Vuppaladadiyam et al. 2018). The shift from carbohydrate to lipid biosynthesis could have occurred due to the alteration in the carbon and energy flux from the dye-irradiance. This further substantiates the increase in growth after day 6. However, to reduce variability, it is advisable to establish continuous systems for consistent nutrient recycling, ensuring the clarity and observability of light-dependent responses. Furthermore, the effect of temperature on growth and metabolite production cannot be overlooked (Supplementary Fig. S10a and b). The variability in the temperatures between the reactors could affect membrane fluidity, and microalgae, in response, can modify the membrane fatty acid composition to maintain homoeostasis (Sathasivam et al. 2019; Najafpour et al. 2022). However, this was not verified in the current study.
Comparison with previous studies
The findings of this study have highlighted the effect of dye-mediated irradiance on C. reinhardtii growth and metabolite production. The results complied with previous studies, showing that excess light conditions increased the accumulation of cellular metabolites (Supplementary Table S3). Nwoba et al. (2021) used monochromatic light filters to down-regulate natural sunlight to the blue and red wavelengths to enhance the biomass productivity of Dunaliella salina in outdoor raceway ponds. The protein content ranged from 156 to 173 mg g-1, while the carbohydrate content was 60, 55, and 49 mg g-1 under red, blue, and control conditions, respectively. The lipid content was 684, 608, and 681 mg g-1 under red, blue, and control conditions, respectively, while the biomass concentrations increased by 2 and 14%, respectively. Similar to the findings of Nwoba et al. (2021), the metabolite content in the current study increased under red and blue/green light when compared to the control. Vadiveloo et al. (2015), integrated a photovoltaic cell with a microalgal cultivation system to provide filtered light spectra to the culture. It was found that blue light yielded the highest biomass productivity per photon and energy supplied (1.93 mg L−1 day−1 [μmol photons m−2 s−1]-1) for Nannochloropsis sp. (MUR266). Also, the lipid content was ~ 60% higher under blue light reinforcing the effect of this wavelength on algal metabolism, (Seo et al. 2015). The authors surmised that this approach could allow for the full exploitation of sunlight and reduce dependence on artificial lighting (Supplementary Table S3).
In terms of feasibility, the use of spectrally selective filters to cultivate algae would be beneficial in the geological regions subjected to higher incident sunlight. Whereas, organic dyes allow for the conversion of incident sunlight, and thus, the concentration of low incident sunlight. For instance, Seo et al. (2015) grew C. vulgaris under the control, DPA and R101 dyes at 50 W m-2 (~ 105 μmol photons m−2 s−1) and found that biomass levels reached 1.0, 1.1, and 1.5 g L-1, respectively. Moreover, the lipid content under R101 and DPA was 22 and 40% higher than the control. Sung et al. (2018), found that when DPA and R101 were used to supplement blue and red spectra, the biomass productivity of N. gaditana increased by 23 – 29%, respectively (data extrapolated from graph). There was no increase in protein and lipid content, however, carbohydrates increased by 14% in DPA-grown cultures (Supplementary Table S3).
Sustainability and future perspectives of dye-assisted algal systems
This study revealed the promising applicability of the LR and R8G dyes as effective spectral converters of incident irradiation, supporting photosynthetic energy utilisation and increasing metabolite yields without increased input of energy in algal systems. This innovative strategy is valuable in enhancing the yield of algal biomass and the production of value-added metabolites. The proposed dye-mediated irradiations are useful for microalgae cultivation and production mechanisms to maintain multiple SDGs. The increased protein and lipids fractions under dye-mediated irradiations (≥ 48% and > 65 g L-1 day-1 in Fig. 6a and c, respectively) can be used for nutritional and functional food/feed products (Kumar and Singh 2019), upholding GOAL 2 “Zero Hunger”. The ability of the algae to accumulate carbohydrates in the cells (reaching ≥ 98 g L-1 day-1, Fig. 6b) would add bio-economic benefit by utilising the algal biomass as a feedstock for biofuels production (Deviram et al. 2020; Ansari et al. 2023). The extracted algal carbohydrates can be applied in bioplastics production, while antioxidant pigment fractions (Fig. 5) may find relevant applications in the food and nutraceutical industries (Kumar et al. 2022). Hence, the potential production of microalgal biomass and bioenergy metabolites would also indirectly meet GOAL 3 “Good Health and Well-being”. The interlinkage between these advantages and the relevant SDGs encourages the widespread application of algal dye-based systems as an ideal example of a real circular bioeconomy (Ansari et al. 2023).
Considerations to be addressed in future research include, the key limitation with the current study is that the cultures were not pre-adapted to the new light (irradiance and spectrum) environment and that the experiments were operated in batch mode. Thus, the algae were acclimating to the spectral changes, the different irradiances, and the changes in nutrients available during the batch culture. This may account for some aspects of the erratic growth curves. Hence, future experiments should ensure the acclimation to the different light spectra and culture conditions. Future research should also elucidate the combined effects of dye spectral conversion and temperature on microalgal growth and metabolite synthesis. It is recommended that immobilisation of the dye/s onto a solid phase such as nanoparticles, the fabrication of PBRs with immobilised dyes in suitable polymers or coating the surfaces of PBRs with fluorescent dye layers could improve their photo-stability and quantum efficiency during microalgal cultivation. This would also ensure dye reusability to maintain an economically feasible cultivation strategy at an industrial scale.
Conclusion
The blue/green and red light emitted from R8G and LR had a positive effect on C. reinhardtii and was associated with increased biomass levels and metabolite productivities. Increases/fluctuations in the metabolic productivities occurred at the expense of others, however, this could allow for targeted metabolite enhancement. The Chlamydomonas early response to the dye-irradiations was to increase growth, chlorophylls and carotenoids, protein, carbohydrate, and lipid productivities in response to photo-acclimation, demonstrating the beneficial effect of the increased dye-mediated irradiance on algal growth. At later stages (> day 4), growth rate slowed (~ 0.2) and there was a reduction in the metabolite and biochemical component production in C. reinhardtii. This study showed that using dyes in microalgal cultivation could be a promising approach for enhancing biomass and metabolite production in sustainable microalgal systems.
Data availability
Data and materials will be made available on request.
References
Abou-Kana MTH (2012) Optical, photophysical, stability and mirrorless lasing properties of novel fluorescein derivative dye in solution. Open J Appl Sci 2:228–235
Almeida AC, Gomes T, Langford K, Thomas KV, Tollefsen KE (2017) Oxidative stress in the algae Chlamydomonas reinhardtii exposed to biocides. Aquat Toxicol 189:50–59
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ansari FA, Nasr M, Rawat I, Bux F (2023) Meeting sustainable development goals (SDGs) through progression of pilot-scale algal system to commercial raceway pond (300,000 L). Biomass Convers Biorefin 13:7663–7676
Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE (2004) Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. BioTechniques 37:598–602
Chia MA, Lombardi AT, da Graca Gama Melao M, Parrish CC, (2015) Combined nitrogen limitation and cadmium stress stimulate total carbohydrates, lipids, protein and amino acid accumulation in Chlorella vulgaris (Trebouxiophyceae). Aquat Toxicol 160:87–95
Delavari Amrei H, Nasernejad B, Ranjbar R, Rastegar S (2014) Spectral shifting of UV-A wavelengths to blue light for enhancing growth rate of cyanobacteria. J Appl Phycol 26:1493–1500
Delgado-Sanchez J-M (2019) Luminescent solar concentrators: Photo-stability analysis and long-term perspectives. Sol Energy Mater Sol Cells 202:110134
Deviram G, Mathimani T, Anto S, Ahamed TS, Ananth DA, Pugazhendhi A (2020) Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J Clean Prod 253:119770
Duarte JH, Costa JAV (2018) Blue light emitting diodes (LEDs) as an energy source in Chlorella fusca and Synechococcus nidulans cultures. Bioresour Technol 247:1242–1245
Esquivel MG, Matos AR, Marques Silva J (2017) Rubisco mutants of Chlamydomonas reinhardtii display divergent photosynthetic parameters and lipid allocation. Appl Microbiol Biotechnol 101:5569–5580
Ezequiel J, Nitschke MR, Laviale M, Serôdio J, Frommlet JC (2022) Concurrent bioimaging of microalgal photophysiology and oxidative. Photosynth Res 155:177–190
Gifuni I, Pollio A, Marzocchella A, Olivieri G (2018) New ultra-flat photobioreactor for intensive microalgal production: the effect of light irradiance. Algal Res 34:134–142
Giridhar Babu A, Wu X, Kabra AN, Kim DP (2017) Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res 23:178–185
Hassan AF, Al-Hamdani AH, Abbas FS (2014) Study the effect of concentration on spectroscopic properties of fluorescein sodium dye in ethanol. J Kufa Phys 6:112–118
Herdean A, Hall C, Hughes D, Kuzhiumparambil U (2023) Temperature mapping of non photochemical quenching in Chlorella. Photosynth Res 155:191–202
Khoobkar Z, Delavari Amrei H (2020) Effect of fluorescent dye positioning and concentration on the growth parameters and lipid content of Chlorella sp. in a flat panel photobioreactor. Biotech Lett 42:1397–1405
Kumar D, Singh B (2019) Algal biorefinery: An integrated approach for sustainable biodiesel production. Biomass Bioenergy 131:105398
Kumar N, Banerjee C, Chang J-S, Shukla P (2022) Valorization of wastewater through microalgae as a prospect for generation of biofuel and high-value products. J Clean Prod 362:132114
Li X, Manuel J, Crunkleton DW, Johannes TW (2021) Effect of blue and red-orange LEDs on the growth and biochemical profile of Chlamydomonas reinhardtii. J Appl Phycol 33:1367–1377
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Ma R, Zhao X, Xie Y, Ho SH, Chen J (2019) Enhancing lutein productivity of Chlamydomonas sp. via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour Technol 275:416–420
Maillard J, Klehs K, Rumble C, Vauthey E, Heilemann M, Furstenberg A (2020) Universal quenching of common fluorescent probes by water and alcohols. Chem Sci 12:1352–62
Mattos ER, Singh M, Cabrera ML, Das KC (2015) Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 81:473–478
Meagher E, Rangsrikitphoti P, Faridi B, Zamzam G, Durnford DG (2021) Photoacclimation to high-light stress in Chlamydomonas reinhardtii during conditional senescence relies on generating pH-dependent, high-quenching centres. Plant Physiol Biochem 158:136–145
Michael C, del Ninno M, Gross M, Wen Z (2015) Use of wavelength-selective optical light filters for enhanced microalgal growth in different algal cultivation systems. Bioresour Technol 179:473–482
Najafpour MM, Shen J-R, Allakhverdiev SI (2022) Natural and artificial photosynthesis: fundamentals, progress, and challenges. Photosynth Res 154:229–231
Nama S, Madireddi SK, Yadav RM, Subramanyam R (2019) Non-photochemical quenching-dependent acclimation and thylakoid organization of Chlamydomonas reinhardtii to high light stress. Photosynth Res 139:387–400
Natali A, Croce R (2015) Characterization of the major light-harvesting complexes (LHCBM) of the green alga Chlamydomonas reinhardtii. PLoS One 10:e0119211
Nawrocki WJ, Liu X, Croce R (2020) Chlamydomonas reinhardtii exhibits de facto constitutive NPQ capacity in physiologically relevant conditions. Plant Physiol 182:472–479
Nordin N, Yusof N, Maeda T, Mustapha NA, Mohd Yusoff MZ, Raja Khairuddin RF (2020) Mechanism of carbon partitioning towards starch and triacylglycerol in Chlorella vulgaris under nitrogen stress through whole-transcriptome analysis. Biomass Bioenergy 138:105600
Nowicka B (2020) Practical aspects of the measurements of non-photochemical chlorophyll fluorescence quenching in green microalgae Chlamydomonas reinhardtii using Open FluorCam. Physiol Plant 168:617–629
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:101433
Nwoba EG, Rohani T, Raeisossadati M, Vadiveloo A, Bahri PA, Moheimani NR (2021) Monochromatic light filters to enhance biomass and carotenoid productivities of Dunaliella salina in raceway ponds. Bioresour Technol 340:125689
Paul K, Gaikwad M, Choudhary P, Mohan N, Pai P, Patil SD, Pawar Y, Chawande A, Banerjee A, Nagle V, Chelliah M, Sapre A, Dasgupta S (2022) Year-round sustainable biomass production potential of Nannochloris sp. in outdoor raceway pond enabled through strategic photobiological screening. Photosynth Res 154:303–328
Ramanna L, Rawat I, Zerrouki D, Bux F (2018) A novel organic dye-based approach to increase photon flux density for enhanced microalgal pigment production. J Clean Prod 198:187–194
Sanchez-Tarre V, Kiparissides A (2021) The effects of illumination and trophic strategy on gene expression in Chlamydomonas reinhardtii. Algal Res 54:102186
Sathasivam R, Radhakrishnan R, Hashem A, Abd Allah EF (2019) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci 26:709–722
Seo YH, Lee Y, Jeon DY, Han J-I (2015) Enhancing the light utilization efficiency of microalgae using organic dyes. Bioresour Technol 181:355–359
Singh R, Upadhyay AK, Singh DV, Singh J, Singh D (2019) Photosynthetic performance, nutrient status and lipid yield of microalgae Chlorella vulgaris and Chlorococcum humicola under UV-B exposure. Curr Res Biotech 1:65–77
Sung MG, Han JI, Lee B, Chang YK (2018) Wavelength shift strategy to enhance lipid productivity of Nannochloropsis gaditana. Biotechnol Biofuels 11:70
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Vuppaladadiyam AK, Prinsen P, Raheem A, Luque R, Zhao M (2018) Microalgae cultivation and metabolites production: a comprehensive review. Biofuel Bioprod Biorefin 12:304–324
Yadav RM, Aslam SM, Madireddi SK, Chouhan N, Subramanyam R (2020) Role of cyclic electron transport mutations pgrl1 and pgr5 in acclimation process to high light in Chlamydomonas reinhardtii. Photosynth Res 146:247–258
Yarnold J, Ross IL, Hankamer B (2016) Photoacclimation and productivity of Chlamydomonas reinhardtii grown in fluctuating light regimes which simulate outdoor algal culture conditions. Algal Res 13:182–194
Zehentbauer FM, Moretto C, Stephen R, Thevar T, Gilchrist JR, Pokrajac D, Richard KL, Kiefer J (2014) Fluorescence spectroscopy of Rhodamine 6G: concentration and solvent effects. Spectrochim Acta A 121:147–151
Zhu J, Chen W, Chen H, Zhang X, He C, Rong J, Wang Q (2016) Improved productivity of neutral lipids in Chlorella sp. A2 by minimal nitrogen supply. Front Microbiol 7:557
Funding
Open access funding provided by Durban University of Technology. This work was supported financially by the Durban University of Technology and the National Research Foundation (South Africa) (UID: 111046).
Author information
Authors and Affiliations
Contributions
Luveshan Ramanna: Conceptualisation, Methodology, Software: Data curation, Writing – Original draft preparation, Reviewing and Editing, Visualisation, Investigation, Validation, and Statistical analyses. Mahmoud Nasr: Software: Data curation, Writing – Reviewing and Editing, Visualisation, Investigation, Validation, and Statistical analyses. Ismail Rawat: Conceptualisation, Supervision, Writing – Reviewing and Editing. Faizal Bux: Supervision, Writing – Reviewing and Editing, Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramanna, L., Nasr, M., Rawat, I. et al. Enhancing Chlamydomonas reinhardtii growth and metabolite biosynthesis using organic dyes as spectral converters. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03238-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03238-6