Abstract
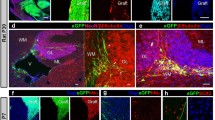
The host response to immunologically incompatible intrastriatal neural grafts was studied using immunohistochemical techniques. Dissociated ventral mesencephalic tissue from embryonic donors of either syngeneic, allogeneic or xenogeneic (mouse) origin was stereotaxically implanted into adult rats. The brains were analysed 4 days, 2 weeks or 6 weeks after grafting with antibodies against the following antigenic structures: major histocompatibility complex (MHC) class I antigens; MHC class II antigens; complement receptor (CR) 3 (marker for microglia and macrophages); helper T-lymphocyte antigen-cluster of differentiation (CD) 4; cytotoxic T-lymphocyte antigen-CD8; tyrosine hydroxylase (TH) (marker for transplanted dopaminergic neurons). The number of surviving TH-positive cells was not different at the various time points in either the syngeneic or allogeneic groups, whereas the xenogeneic cells were all rejected by 6 weeks.
The host reactions were similar in character in the syngeneic and allogeneic groups. At 4 days after implantation, there were increased levels of expression of MHC class I and II antigens. In and around the grafts, there were cellular infiltrates consisting of activated microglia, macrophages, CD4- and CD8-positive lymphocytes. At 6 weeks, MHC expression was reduced and the cellular infiltrates had subsided with only low numbers of activated microglia cells and CD8-positive lymphocytes remaining. In the xenogeneic group, at 4 days, some grafts contained cavities, possibly reflecting acute rejection. At later stages, the xenografts were heavily infiltrated by macrophages, activated microglial cells and T-lymphocytes, and at 6 weeks all the xenografts were rejected.
Taken together, the results suggest that there is an inflammation caused by the implantation process which leads to an accumulation of host defence cells. This, in turn, leads to increased MHC expression in and around the grafts. In syngeneic grafts, these reactions are short lasting and weak; for allografts slightly more pronounced and longer lasting than syngeneic grafts, but not sufficient to cause rejection. For xenografts, the reactions are more intense and lead to transplant rejection. Thus, a strong sustained inflammatory response may be an important determinator for the failure of histoincompatible neural grafts. It can be speculated that a short-term anti-inflammatory treatment of graft recipients may be a sufficient immunosuppressive regimen to allow long-term graft survival.
Similar content being viewed by others
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Abrous N, Guy J, Vigny A, Calas A, Le Moal M, Herman JP (1988) Development of intracerebral dopaminergic grafts: a combined immunohistochemical and autoradiographic study of its time-course and environmental influences. J Comp Neurol 273:26–41
Backes M, Lund RD, Lagenaur CF, Kunz HW, Gill TJ III (1990) Cellular events associated with peripherally induced rejection of mature neural xenografts placed into neonatal rat brains. J Comp Neurol 295:428–437
Barker CF, Billingham RE (1977) Immunologically privileged sites. Adv Immunol 25:1–54
Björklund A, Stenevi U, Dunnett SB, Gage FH (1982) Cross-species neural grafting in a rat model of Parkinson's disease. Nature 298:652–654
Brundin P, Strecker RE (1991) Preparation and intracerebral grafting of dissociated fetal brain tissue in rats. In: Conn PM (eds) Lesions and transplantation (Methods in neurosciences, vol 7). Academic Press, San Diego, pp 305–326
Brundin P, Nilsson OG, Gage FH, Björklund A (1985) Cyclosporin A increases survival of cross-species intrastriatal grafts of embryonic dopamine-containing neurons. Exp Brain Res 60:204–208
Brundin P, Widner H, Nilsson OG, Strecker RE, Björklund A (1989) Intracerebral xenografts of dopamine neurons: the role of immunosuppression and the blood-brain barrier. Exp Brain Res 75:195–207
Cross AH, Canella B, Brosnan CF, Raine CS (1990) Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic and relapsing experimental allergic encephalitis. Lab Invest 63:162–170
Daniloff JK, Low WC, Bodony RP, Wells J (1985) Cross-species neural transplants of embryonic septal nuclei to the hippocompal formation of adult rats. Exp Brain Res 59:73–82
Date I, Kawamura K, Nakashima H (1988) Histological signs of immune reactions against allogeneic solid fetal neural grafts in the mouse cerebellum depend on the MHC locus. Exp Brain Res 73:15–22
Doucet G, Brundin P, Descarries L, Björklund A (1990) Effect of prior denervation on survival and fiber outgrowth from intrastriatal fetal mesencephalic grafts. Eur J Neurosci 2:279–290
Duan W-M, Widner H, Brundin P (1993) Sequential intrastriatal grafting of allogeneic embryonic dopamine-rich neuronal tissue in adult rats: will the second graft be rejected? Neuroscience 57:261–274
Duan W-M, Widner H, Frodl EM, Brundin P (1995) Immune reactions following systemic immunization prior or subsequent to intrastriatal transplantation of allogeneic mesencephalic tissue in adult rats. Neuroscience 64:629–641
Fierz W, Endler B, Reske K, Wekerle H, Fontana A (1985) Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune Interferon and its effect on antigen presentation. J Immunol 134:3785–3793
Finsen BR, Poulsen PH, Zimmer J (1988) Xenografting of fetal mouse hippocampal tissue to the brain of adult rats: effects of cyclosporin A treatment. Exp Brain Res 70:117–133
Finsen BR, Sørensen T, Castellano B, Pedersen EB, Zimmer J (1991) Leukocyte infiltration and glial reactions in xenografts of mouse brain tissue undergoing rejection in the adult rat brain. A light and electron microscopical immunocytochemical study. J Neuroimmunol 32:159–183
Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A (1987) Antigen presentation and tumor cytotoxicity by interferon-γ-treated microglial cells. Eur J Immunol 17:1271–1278
Hart DNJ, Fabre JW (1981) Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissue of rat heart and other tisues, but not brain. J Exp Med 154:347–361
Head JR, Griffin WS (1985) Functional capacity of solid tissue transplants in the brain: evidence for immunological privilege. Proc R Soc Lond B 224:375–387
Hickey WF, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239:290–292
Inoue H, Kohsaka S, Yoshida K, Ohtani M, Toya S, Tsukada Y (1985) Cyclosporin A enhances the survivability of mouse cerebral cortex grafted into the third ventricle of rat brain. Neurosci Lett 54:85–90
Isono M, Poltorak M, Kulaga H, Adams AJ, Freed WJ (1993) Certain host-donor rat strain combinations do not reject brain allografts after systemic sensitization. Exp Neurol 122:48–56
Kohsaka S, Shinozaki T, Nakano Y, Takei K, Toya S, Tsukada Y (1989) Expression of Ia antigen on vascular endothelial cells in mouse cerebral tissue grafted into the third ventricle of rat brain. Brain Res 484:340–347
Lafferty KJ, Prowse SJ, Simeonovic CJ (1983) Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol 1:143–173
Lampson LA (1987) Molecular bases of the immune response to neural antigens. Trends Neurosci 10:211–216
Lawrence JM, Morris RJ, Wilson DJ, Raisman G (1990) Mechanisms of allograft rejection in the rat brain. Neuroscience 37:431–462
Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150:2659–2667
Lindvall O (1991) Prospects of transplantation in human neurodegenerative diseases. Trends Neursosci 14:376–384
Male D, Pryce G (1989) Induction of Ia molecules on brain endothelium is related to susceptibility to experimental allergic encephalomyelitis. J Neuroimmunol 21:87–90
Mason DW, Charlton HM, Jones AJ, Lavy CB, Puklavec M, Simmonds SJ (1986) The fate of allogeneic and xenogeneic neuronal tissue transplanted into the third ventricle of rodents. Neuroscience 19:685–694
Matsumoto Y, Hara N, Tanaka R, Fujiwara M (1986) Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis with special reference to Ia-positive cells with dendritic morphology. J Immunol 136:3668–3676
Nakao N, Frodl EM, Duan W-M, Widner H, Brundin P (1994) Lazaroids improve the survival of grafted rat embryonic dopamine neurons. Proc Natl Acad Sci USA 91:12408–12412
Nakshima H, Kawamura K, Date I (1988) Immunological reaction and blood-brain barrier in mouse-to-rat cross-species neural graft. Brain Res 475:232–243
Nicholas MK, Antel JP, Stefansson K, Arnason BGW (1987) Rejection of fetal neocortical neural transplants by H-2 incompatible mice. J Immunol 139:2275–2283
Panek RB, Moses H, Ting JPY, Benveniste EN (1992) Tumor necrosis factor-α response elements in the HLA-DRA promotor: identification of a tumor necrosis factor α-induced DNA-protein complex in astrocytes. Proc Natl Acad Sci USA 89:11518–11522
Perry VH, Hume DA, Gordon S (1985) Immunohistochemical localizations of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15:313–326
Pollack IF, Lund RD, Rao K (1990) MHC antigen expression and cellular response in spontaneous and induced rejection of neural xenografts. Prog Brain Res 82:129–140
Poltorak M, Freed WJ (1989) Immunological reactions induced by intracerebral transplantation: evidence that host microglial but not astroglia are the antigen-presenting cells. Exp Neurol 103:222–233
Poltorak M, Freed WJ (1991) BN rats do not reject F344 brain allografts even after systemic sensitization. Ann Neurol 29:377–388
Pryce G, Male DK, Sedgwick J (1989) Antigen presentation in the brain: brain endothelial cells are poor simulators of T cell proliferation. Immunology 66:207–212
Rao K, Lund RD, Kunz HW, Gill TJ III (1989) The role of MHC and non-MHC antigens in the rejection of intracerebral allogeneic neural grafts. Transplantation 48:1018–1021
Sauer H, Brundin P (1991) Effects of cool storage on survival and function of intrastriatal ventral mesencephalic grafts. Restor Neurol Neurosci 2:123–135
Sedgwick JD, Mossner R, Schwender S, ter Meulen V (1991) Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. J Exp Med 173:1235–1246
Sloan DJ, Baker BJ, Puklavec M, Charlton HM (1990) The effect of site of transplantation and histocompatibility differences on the survival of neural tissue transplanted to the CNS of different inbred rat strains. Prog Brain Res 82:141–152
Sobel RA, Blanchette BW, Bhan AK, Colvin RB (1984) The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J Immunol 132:2402–2407
Steiniger B, Van der Meide PH (1988) Rat ependyma and microglia cells express class II MHC antigens after intravenous infusion of recombinant gamma interferon. J Neuroimmol 19:111–118
Takei K, Nakano Y, Shinozaki T, Toya S, Tsukada Y, Kohsaka S (1990) Immunological rejection of grafted tissue in xenogeneic neural transplantation. Prog Brain Res 82:103–109
Traugott U, Scheinberg LC, Raine CS (1985) On the presence of Ia-positive endothelial cells and astrocytes in multiple sclerosis lesions and its relevance to antigen presentation. J Neuroimmunol 8:1–14
Wekerle H, Linington C, Lassman H, Meyerman R (1986) Cellular immune reactivity within the CNS. Trends Neurosci 9:271–277
Widner H, Brundin P (1988) Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res Rev 13:287–324
Widner H, Brundin P (1993) Sequential intracerebral transplantation of allogeneic and syngeneic fetal dopamine-rich neuronal tissue in adult rats: will the first graft be rejected? Cell Transplantation 2:307–317
Widner H, Brundin P, Björklund A, Möller E (1989) Survival and immunogenicity of dissociated allogeneic fetal neural dopamine-rich grafts when implanted into the brains of adult mice. Exp Brain Res 76:187–197
Wood MJA, Sloan DJ, Dallman MJ, Charlton HM (1992) A monoclonal antibody to the interleukin-2 receptor enhances the survival of neural allografts — a time-course study. Neuroscience 49:409–418
Young MJ, Rao K, Lund RD (1989) Integrity of the blood-brain barrier in retinal xenografts is correlated with the immunological status of the host. J Comp Neurol 283:107–117
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duan, WM., Widner, H. & Brundin, P. Temporal pattern of host responses against intrastriatal grafts of syngeneic, allogeneic or xenogeneic embryonic neuronal tissue in rats. Exp Brain Res 104, 227–242 (1995). https://doi.org/10.1007/BF00242009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00242009