Summary
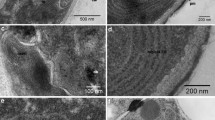
A detailed investigation into the ultrastructure of the pea aphid mycetocytes and their contained symbiotes and organelles was carried out with the transmission electron microscope. The most striking observation was the presence of small vesicles in the space between the primary symbiote cell wall and membrane envelope (outer membrane space). The vesicles appear to form by a budding process at the outer cell wall layer. Subsequently, the vesicles, we suggest, may move out into the mycetocyte cytoplasm via a similar budding of the membrane envelope. The Golgi apparatus was found to be an important structural component of the primary mycetocyte; it is continuous with the rough endoplasmic reticulum and the latter, in turn, appears to be closely connected to the primary symbiote membrane envelope. This may be of functional significance. A number of other organelles not previously described in mycetocytes were found, including transparent vacuoles, granular bodies, multivesicular bodies and microfilaments. The chemical composition of the various vesicles and organelles is unknown at present.
Similar content being viewed by others
References
Akey, D. H.: Nutrition and culture of the pea aphid, Acyrthosiphon pisum, on defined diets. Ph. D. Thesis, University of Wisconsin (1972)
Akey, D. H., Beck, S. D.: Continuous rearing of the pea aphid, Acyrthosiphon pisum, on a holidic diet. Ann. entomol. Soc. Amer. 64, 353–356 (1971)
Buchner, P.: Endosymbioses of animals with plant microorganisms. New York: Interscience 1965
Burge, R. E., Draper, J. C.: The structure of the cell wall of the gram-negative bacterium Proteus vulgaris. III. A lipopolysaccharide “unit membrane.” J. molec. Biol. 28, 205–210 (1967)
Costerton, J. W., Ingram, J. M., Cheng, K. J.: Structure and function of the cell envelope of gram-negative bacteria. Bact. Rev. 38, 87–110 (1974)
Dadd, R. H., Krieger, D. L.: Continuous rearing of aphids of the Aphis fabae complex on sterile synthetic diets. J. econ. Ent. 60, 1512–1514 (1967)
Erhardt, P.: Die Wirkung verschiedener Spurenelemente auf Wachstum, Reproduktion und Symbionten von Neomyzus circumflexus Buckt (Aphididae, Homoptera, Insecta), bei künstlicher Ernährung. Z. vergl. Physiol. 58, 47–75 (1968)
Erhardt, P.: Die Rolle von Methionin, Cystein, Cystin und Sulfat bei der künstlichen Ernährung von Neomyzus (Aulacortum) circumflexus Buckt (Aphididae, Homoptera, Insecta). Biol. Zbl. 88, 335–348 (1969)
Griffiths, G. W., Beck, S. D.: Intracellular symbiotes of the pea aphid, Acyrthosiphon pisum. J. Insect. Physiol. 19, 75–84 (1973)
Griffiths, G. W., Beck, S. D.: Effects of antibiotics on intracellular symbiotes in the pea aphid, Acyrthosiphon pisum. Cell. Tiss. Res. 148, 287–300 (1974)
Hinde, R.: Structural and physiological studies of the mycetome symbiotes of aphids. Ph. D. Thesis, University of Sydney (1970)
Jamieson, J. E., Palade, G. E.: Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J. Cell Biol. 34, 577–596 (1967)
Locke, M. J., McMahon, J. T.: The origin and fate of microbodies in the fat body of an insect. J. Cell Biol. 48, 61–78 (1971)
McLean, D. L., Houk, E. J.: Phase contrast and electron microscopy of the mycetocytes and symbiotes of the pea aphid, Acyrthosiphon pisum. J. Insect. Physiol. 19, 625–635 (1973)
Palade, G. E.: A study of fixation for electron microscopy. J. exp. Med. 95, 285–298 (1952)
Perdue, J. F.: The distribution, ultrastructure and chemistry of microfilaments in cultured chick embryo fibroblasts. J. Cell Biol. 58, 265–283 (1973)
Reynolds, E. S.: The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963)
Rothfield, L., Pearlman-Kolthencz, M.: Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-Phospholipid-Protein complex excreted by living bacteria. J. molec. Biol. 44, 477–492 (1969)
Thompson, M. J., Svoboda, J. A., Kaplanig, J. N., Robbins, W. E.: Metabolic pathways of steroids in insects. Proc. roy. Soc. B 180, 203–221 (1972)
Author information
Authors and Affiliations
Additional information
Research supported by the College of Agricultural and Life Sciences, University of Wisconsin, and by a research grant (GB 31840 X) from the National Science Foundation.
Many thanks to Dr. E. J. Houk, Dr. G. A. DeZoeten, and Dr. E. H. Newcomb for their valuable ideas and suggestions and to Mr. Gary Gaard for technical assistance.
Rights and permissions
About this article
Cite this article
Griffiths, G.W., Beck, S.D. Ultrastructure of pea aphid mycetocytes: Evidence for symbiote secretion. Cell Tissue Res. 159, 351–367 (1975). https://doi.org/10.1007/BF00221782
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00221782