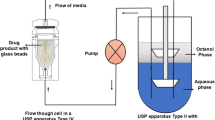
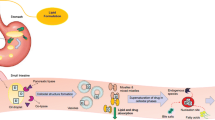
Abstract
There is increasing interest in the use of lipid-based formulations for the delivery of poorly water-soluble drugs. After ingestion of the formulation, exposure to the gastrointestinal environment results in dispersion and digestion processes, leading to the production of amphiphilic digestion products that form self-assembled structures in the aqueous environment of the intestine. These structures are crucial for the maintenance of drug in a solubilized state prior to absorption. This review describes the structural techniques used to study such systems, the structures formed in assembled ‘equilibrium’ compositions where components are combined in expected ratios representative of the endpoint of digestion, structures formed using dynamic in vitro ‘non-equilibrium’ digestion models where the composition and hence structures present change over time and observations from ex vivo aspirated samples. Possible future directions towards an improved understanding of the structural aspects of lipid digestion are proposed.












Similar content being viewed by others
References
Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25(1):103–28.
Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179–88.
Kossena GA et al. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water-soluble drugs. J Pharm Sci. 2003;92(3):634–48.
Kossena GA et al. Influence of the intermediate digestion phases of common formulation lipids on the absorption of a poorly water-soluble drug. J Pharm Sci. 2005;94(3):481–92.
Sek L, Porter CJH, Charman WN. Characterisation and quantification of medium-chain and long-chain triglycerides and their in vitro digestion products by HPTLC coupled with in situ densitometric analysis. J Pharm Biomed Anal. 2001;25(3–4):651–61.
Sek L et al. Evaluation of the in vitro digestion profiles of long and medium-chain glycerides and the phase behaviour of their lipolytic products. J Pharm Pharmacol. 2002;54(1):29–41.
Kaukonen A et al. Drug solubilization behaviour during in vitro digestion of suspension formulations of poorly water-soluble drugs in triglyceride lipids. Pharm Res. 2004;21(2):254–60.
Kaukonen AM et al. Drug solubilization behaviour during in vitro digestion of simple triglyceride lipid solution formulations. Pharm Res. 2004;21(2):245–53.
Zangenberg NH et al. A dynamic in vitro lipolysis model II: evaluation of the model. Eur J Pharm Sci. 2001;14(3):237–44.
Zangenberg NH et al. A dynamic in vitro lipolysis model I. Controlling the rate of lipolysis by continuous addition of calcium. Eur J Pharm Sci. 2001;14(2):115–22.
Salentinig S et al. Transitions in the internal structure of lipid droplets during fat digestion. Soft Matter. 2011;7(2):650–61.
Borovicka J et al. Regulation of gastric and pancreatic lipase secretion by CCK and cholinergic mechanisms in humans. Am J Physiol Gastrointest Liver Physiol. 1997;273(2):G374–80.
Moreau H et al. Immunocytolocalization of human gastric lipase in chief cells of the fundic mucosa. Histochemistry. 1989;91(5):419–23.
Thomson ABR et al. Intestinal aspects of lipid absorption: in review. Can J Physiol Pharmacol. 1989;67(3):179–91.
Hamosh M et al. Fat digestion in the newborn: characterisation of lipase in gastric aspirates of premature and term infants. J Clin Investig. 1981;67(3):838–46.
Chapus C et al. Mechanism of pancreatic lipase action. 1. Interfacial activation of pancreatic lipase. Biochemistry. 1976;15(23):4980–7.
Widmaier EP. e.a., Vander's human physiology: the mechanisms of body function. 10th ed. New York: McGraw-Hill; 2006.
Persson E et al. Simultaneous assessment of lipid classes and bile acids in human intestinal fluid by solid-phase extraction and HPLC methods. J Lipid Res. 2007;48(1):242–51.
Kalantzi L et al. Canine intestinal contents vs. simulated media for the assessment of solubility of two weak bases in the human small intestinal contents. Pharm Res. 2006;23(6):1373–81.
Russell TL et al. Upper gastrointestinal ph in 79 healthy, elderly, North-American men and women. Pharm Res. 1993;10(2):187–96.
Evans DF et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–41.
Salentinig S, Sagalowicz L, Glatter O. Self-assembled structures and pK(a) value of oleic acid in systems of biological relevance. Langmuir. 2010;26(14):11670–9.
Vinarov Z et al. In vitro study of triglyceride lipolysis and phase distribution of the reaction products and cholesterol: effects of calcium and bicarbonate. Food Funct. 2012;3(11):1206–20.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimising the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Reis P et al. Competition between lipases and monoglycerides at interfaces. Langmuir. 2008;24(14):7400–7.
Reis P et al. Adsorption of polar lipids at the water–oil interface. Langmuir. 2008;24(11):5781–6.
Chakraborty S et al. Lipid: an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73(1):1–15.
Horter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 1997;25(1):3–14.
Kleberg K et al. Biorelevant media simulating fed state intestinal fluids: colloid phase characterization and impact on solubilization capacity. J Pharm Sci. 2010;99(8):3522–32.
Porter CJH, Charman WN. In vitro assessment of oral lipid-based formulations. Adv Drug Deliv Rev. 2001;50:S127–47.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and 'self-microemulsifying' drug delivery systems. Eur J Pharm Sci. 2000;11:S93–8.
Porter CJH et al. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm Res. 2004;21(8):1405–12.
Warren DB et al. Real time evolution of liquid crystalline nanostructure during the digestion of formulation lipids using synchrotron small-angle x-ray scattering. Langmuir. 2011;27(15):9528–34.
Porter CJH, Charman WN. Intestinal lymphatic drug transport: an update. Adv Drug Deliv Rev. 2001;50(1–2):61–80.
Folmer BM et al. Monocomponent hexa- and dodecaethylene glycol succinyl-tocopherol esters: self-assembly structures, cellular uptake and sensitivity to enzyme hydrolysis. Biochem Pharmacol. 2009;78(12):1464–74.
Tan A et al. Hybrid nanomaterials that mimic the food effect: controlling enzymatic digestion for enhanced oral drug absorption. Angew Chem Int Ed. 2012;51(22):5475–9.
Tan A et al. Silica nanoparticles to control the lipase-mediated digestion of lipid-based oral delivery systems. Mol Pharm. 2010;7(2):522–32.
Small DM. A classification of biologic lipids based upon their interaction in aqueous systems. J Am Oil Chem Soc. 1968;45(3):108.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Gao P et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98.
Gao P, Shi Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14(4):703–13.
Thomas N et al. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J Control Release. 2012;160(1):25–32.
Kaasgaard T, Drummond CJ. Ordered 2-D and 3-D nanostructured amphiphile self-assembly materials stable in excess solvent. Phys Chem Chem Phys. 2006;8(43):4957–75.
Fong WK, Hanley T, Boyd BJ. Stimuli responsive liquid crystals provide 'on-demand' drug delivery in vitro and in vivo. J Control Release. 2009;135(3):218–26.
Yaghmur A et al. Effects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein-oil systems. Langmuir. 2010;26(2):1177–85.
Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Faraday Trans Ii. 1976;72:1525–68.
Yaghmur A et al. Emulsified microemulsions and oil-containing liquid crystalline phases. Langmuir. 2005;21(2):569–77.
Sagalowicz L et al. Monoglyceride self-assembly structures as delivery vehicles. Trends Food Sci Technol. 2006;17(5):204–14.
Shearman GC et al. Inverse lyotropic phases of lipids and membrane curvature. J Phys Condens Matter. 2006;18(28):S1105–24.
Seddon JM et al. Inverse micellar phases of phospholipids and glycolipids. Phys Chem Chem Phys. 2000;2(20):4485–93.
Schwarz US, Gompper G. Bending frustration of lipid-water mesophases based on cubic minimal surfaces. Langmuir. 2001;17(7):2084–96.
Seddon JM et al. An Fd3m lyotropic cubic phase in a binary glycolipid/water system. Langmuir. 1996;12(22):5250–3.
Patton JS, Carey MC. Watching fat digestion. Science. 1979;204(4389):145–8.
Staggers JE et al. Physical–chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of health adult human beings. Biochemistry. 1990;29(8):2028–40.
Kossena GA et al. A novel cubic phase of medium-chain lipid origin for the delivery of poorly water-soluble drugs. J Control Release. 2004;99(2):217–29.
Patton JS et al. The light-microscopy of triglyceride digestion. Food Microstruct. 1985;4(1):29–41.
Rigler MW, Honkanen RE, Patton JS. Visualisation by freeze fracture, in vitro and in vivo, of the products of fat digestion. J Lipid Res. 1986;27(8):836–57.
Rigler MW, Patton JS. The production of liquid-crystalline product phases by pancreatic lipase in the absence of bile salts - a freeze-fracture study. Biochimica Et Biophysica Acta. 1983;751(3):444–54.
Hernell O, Staggers JE, Carey MC. Physical–chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2 Phase-analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29(8):2041–56.
Weibull C, Christiansson A, Carlemalm E. Extraction of membrane-lipids during fixation, dehydration and embedding of acholeplasma-laidlawii-cells for electron-microscopy. J Microsc Oxford. 1983;129:201–7.
Dermier GB. An autoradiographic and biochemical study of oleic acid absorption by intestinal slices including determinations of lipid loss during preparation for electron microscopy. J Ultrastruct Res. 1968;22(3–4):312.
Muller M, Meister N, Moor H. Freezing in a propane jet and its application in freeze-fracturing. Mikroskopie. 1980;36(5–6):129–40.
Danino D. Cryo-TEM of soft molecular assemblies. Curr Opin Colloid Interface Sci. 2012;17(6):316–29.
Gustafsson J et al. Phase behavior and aggregate structure in aqueous mixtures of sodium cholate and glycerol monooleate. J Colloid Interface Sci. 1999;211(2):326–35.
Fatouros DG et al. Structural development of self nano emulsifying drug delivery systems (SNEDDS) during in vitro lipid digestion monitored by small-angle x-ray scattering. Pharm Res. 2007;24(10):1844–53.
Fatouros DG, Bergenstahl B, Mullertz A. Morphological observations on a lipid-based drug delivery system during in vitro digestion. Eur J Pharm Sci. 2007;31(2):85–94.
Phan S, H.A., Mulet X, Waddington L, Prestidge C, Boyd B.J. Structural aspects of digestion of medium chain triglycerides studied in real time using sSAXS and cryo-TEM. Pharmaceutical Research; 2013. In press.
Yaghmur A, Glatter O. Characterization and potential applications of nanostructured aqueous dispersions. Adv Colloid Interface Sci. 2009;147–148:333–42.
Tan G et al. Cryo-field emission scanning electron microscopy imaging of a rigid surfactant mesophase. Langmuir. 2008;24(19):10621–4.
Fong W-K et al. Controlling the nanostructure of gold nanorod–lyotropic liquid-crystalline hybrid materials using near-infrared laser irradiation. Langmuir. 2012;28(40):14450–60.
Rizwan SB et al. Characterisation of bicontinuous cubic liquid crystalline systems of phytantriol and water using cryo field emission scanning electron microscopy (cryo FESEM). Micron. 2007;38(5):478–85.
Boyd BJ et al. Self-assembled geometric liquid-crystalline nanoparticles imaged in three dimensions: hexosomes are not necessarily flat hexagonal prisms. Langmuir. 2007;23(25):12461–4.
Svaerd M et al. Micelles, vesicles and liquid crystals in the monoolein-sodium taurocholate-water system: phase behavior, NMR, self-diffusion, and quasi-elastic light scattering studies. J Phys Chem. 1988;92(8):2261–70.
Koppel DE. Analysis of macromolecular polydispersity in intensity correlation spectroscopy—method of cumulants. J Chem Phys. 1972;57(11):4814.
Berne BJP. R, Dynamic Light Scattering. New York: Wiley-Interscience; 1976.
Hyde S. Handbook of Applied Surface and Colloid Chemistry, ed. K. Holmberg: John Wiley & Sons, Ltd; 2001. 299–332.
Bragg WL, Thomson JJ. The diffraction of short electromagnetic waves by a crystal. Proc Camb Philos Soc. 1914;17:43–57.
Seddon J.M et al. Structural Studies of Liquid Crystals by X-Ray Diffraction, in Handbook of Liquid Crystals Set. Wiley-VCH Verlag GmbH; 2008. p. 635–679.
Borne J, Nylander T, Ehan A. Effect of lipase on different lipid liquid crystalline phases formed by oleic acid-based acylglycerols in aqueous systems. Langmuir. 2002;18(23):8972–81.
Kossena GA et al. Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid-based delivery systems: a phase diagram approach. J Pharm Sci. 2004;93(2):332–48.
Westerman PW. Physicochemical characterization of a model digestive mixture by 2H NMR. J Lipid Res. 1995;36(12):2478–92.
Boetker J et al. Structural elucidation of rapid solution-mediated phase transitions in pharmaceutical solids using in situ synchrotron SAXS/WAXS. Mol Pharm. 2012;9(9):2787–91.
Fatouros DG et al. Physicochemical characterization of simulated intestinal fed-state fluids containing lyso-phosphatidylcholine and cholesterol. Dissolution Technol. 2009;16(3):47–50.
Caffrey M. The study of lipid phase-transition kinetics by time-resolved x-ray-diffraction. Annu Rev Biophys Biophys Chem. 1989;18:159–86.
Lopez-Rubio A, Gilbert EP. Neutron scattering: a natural tool for food science and technology research. Trends Food Sci Technol. 2009;20(11–12):576–86.
Small DM, Penkett SA, Chapman D. Studies on simple and mixed bile salt micelles by nuclear magnetic resonance spectroscopy. Biochimica Et Biophysica Acta. 1969;176(1):178.
Ljusbergwahren H, Larsson K. A Raman-spectroscopy study of mixed bile salt–monoglyceride micelles. Chem Phys Lipids. 1981;28(1):25–32.
Day JP et al. Label-free imaging of lipophilic bioactive molecules during lipid digestion by multiplex coherent anti-stokes Raman scattering microspectroscopy. J Am Chem Soc. 2010;132(24):8433–9.
Rube A, Klein S, Mader K. Monitoring of in vitro fat digestion by electron paramagnetic resonance spectroscopy. Pharm Res. 2006;23(9):2024–9.
Muller K. Structural aspects of bile salt-lecithin mixed micelles. Hepatology. 1984;4(5):S134–7.
Walter A et al. Intermediate structures in the cholate–phosphatidylcholine vesicle micelle transition. Biophys J. 1991;60(6):1315–25.
Hofmann AF. Molecular association in fat digestion—interaction in bulk of monoolein oleic acid and sodium oleate with dilute micellar bile salt solutions. Adv Chem Ser. 1968;84:53.
Fatouros DG et al. Colloidal structures in media simulating intestinal fed state conditions with and without lipolysis products. Pharm Res. 2009;26(2):361–74.
Borne J, Nylander T, Khan A. Phase behavior and aggregate formation for the aqueous monoolein system mixed with sodium oleate and oleic acid. Langmuir. 2001;17(25):7742–51.
Borne J, Nylander T, Khan A. Microscopy, SAXD, and NMR studies of phase behavior of the monoolein–diolein water system. Langmuir. 2000;16(26):10044–54.
Larsson K, Gabrielsson K, Lundberg B. Phase behavior of some aqueous systems involving monoglycerides, cholesterol and bile acids. J Sci Food Agric. 1978;29(10):909–14.
Lindstrom M et al. Aqueous lipid phases of relevance to intestinal fat digestion and absorption. Lipids. 1981;16(10):749–54.
Hjelm RP et al. Structure of conjugated bile salt-fatty acid-monoglyceride mixed colloids: studies by small-angle neutron scattering. J Phys Chem B. 2000;104(2):197–211.
Cohen DE, Angelico M, Carey MC. Structural alterations in lecithin-cholesterol vesicles following interactions with monomeric and micellar bile salts: physical–chemical basis for sub-selection of biliary lecithin species and aggregative states of biliary lipids during bile formation. J Lipid Res. 1990;31(1):55–70.
Embleton JK, Pouton CW. Structure and function of gastrointestinal lipases. Adv Drug Deliv Rev. 1997;25(1):15–32.
Qiu H, Caffrey M. The phase diagram of the monoolein/water system: metastability and equilibrium aspects. Biomaterials. 2000;21(3):223–34.
Lutton ES. Phase behavior of aqueous systems of monoglycerides. J Am Oil Chem Soc. 1965;42(12):1068.
Borne J, Nylander T, Khan A. Vesicle formation and other structures in aqueous dispersions of monoolein and sodium oleate. J Colloid Interface Sci. 2003;257(2):310–20.
Hofmann AF, Borgstrom B. Intraluminal phase of fat digestion in man—lipid content of micellar + oil phases of intestinal content obtained during fat digestion + absorption. J Clin Investig. 1964;43(2):247.
Mazer NA, Benedek GB, Carey MC. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt–lecithin solutions. Biochemistry. 1980;19(4):601–15.
Schurtenberger P, Mazer N, Kanzig W. Micelle to vesicle transition in aqueous solutions of bile-salt and lecithin. J Phys Chem. 1985;89(6):1042–9.
Long MA, Kaler EW, Lee SP. Structural characterization of the micelle–vesicle transition in lecithin bile salt solutions. Biophys J. 1994;67(4):1733–42.
Hjelm RP, Thiyagarajan P, Alkan H. A small-angle neutron-scattering study of the effects of dilution on particle morphology in mixtures of glycocholate and lecithin. J Appl Crystallogr. 1988;21:858–63.
Vinson PK, Talmon Y, Walter A. Vesicle–micelle transition of phosphatidylcholine and octyl glucoside elucidated by cryo-transmission electron microscopy. Biophys J. 1989;56(4):669–81.
Armand M et al. Physicochemical characteristics of emulsions during fat digestion in human stomach and duodenum. Am J Physiol Gastrointest Liver Physiol. 1996;271(1):G172–83.
Schulze K. Imaging and modelling of digestion in the stomach and the duodenum. Neurogastroenterol Motil. 2006;18(3):172–83.
Kalantzi L et al. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76.
Christensen JO et al. Solubilisation of poorly water-soluble drugs during in vitro lipolysis of medium- and long-chain triacylglycerols. Eur J Pharm Sci. 2004;23(3):287–96.
Porter CJH et al. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J Pharm Sci. 2004;93(5):1110–21.
Gargouri Y, Moreau H, Verger R. Gastric lipases: biochemical and physiological studies. Biochimica Et Biophysica Acta. 1989;1006(3):255–71.
Dressman JB et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7(7):756–61.
Larsen AT, Sassene P, Mullertz A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant-based drug delivery systems. Int J Pharm. 2011;417(1–2):245–55.
Williams HD et al. Toward the establishment of standardised in vitro tests for lipid-based formulations, part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J Pharm Sci. 2012;101(9):3360–80.
Dong YD, Boyd BJ. Applications of X-ray scattering in pharmaceutical science. Int J Pharm. 2011;417(1–2):101–11.
Mullertz A et al. Insights into intermediate phases of human intestinal fluids visualised by atomic force microscopy and cryo-transmission electron microscopy ex vivo. Mol Pharm. 2012;9(2):237–47.
Armand M et al. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am J Clin Nutr. 1999;70(6):1096–106.
Acknowledgments
Funding is acknowledged from the Australian Research Council under the Discovery Projects scheme DP120104032. BJB is a recipient of an Australian Research Council Future Fellowship (FT120100697).
Conflict of interest
The authors collectively declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phan, S., Salentinig, S., Prestidge, C.A. et al. Self-assembled structures formed during lipid digestion: characterization and implications for oral lipid-based drug delivery systems. Drug Deliv. and Transl. Res. 4, 275–294 (2014). https://doi.org/10.1007/s13346-013-0168-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-013-0168-5