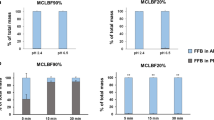
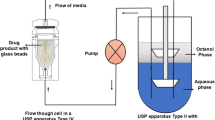
ABSTRACT
An increasing number of newly discovered drugs are poorly water-soluble and the use of natural and synthetic lipids to improve the oral bioavailability of these drugs by utilizing the digestion pathway in-vivo has proved an effective formulation strategy. The mechanisms responsible for lipid digestion and drug solubilisation during gastrointestinal transit have been explored in detail, but the implications of drug precipitation beyond the potential adverse effect on bioavailability have received attention only in recent years. Specifically, these implications are that different solid forms of drug on precipitation may affect the total amount of drug absorbed in-vivo through their different physico-chemical properties, and the possibility that the dynamic environment of the small intestine may afford re-dissolution of precipitated drug if present in a high-energy form. This review describes the events that lead to drug precipitation during the dispersion and digestion of lipid based formulations, common methods used to inhibit precipitation, as well as conventional and newly emerging characterization techniques for studying the solid state form of the precipitated drug. Moreover, selected case studies are discussed where drug precipitation has ensued from the digestion of lipid based formulations, as well as the apparent link between drug ionisability and altered solid forms on precipitation, culminating in a discussion about the importance of the solid form on precipitation with relevance to the total drug absorbed.




Similar content being viewed by others
Abbreviations
- LBDDS:
-
Lipid based drug delivery systems
- BCS:
-
Biopharmaceutics classification system
- LFCS:
-
Lipid formulation classification system
- SRM :
-
Maximum supersaturation ratio
- XRD:
-
X-ray diffraction
- CPLM:
-
Crossed polarized light microscopy
- DSC:
-
Differential scanning calorimetry
- FTIR:
-
Fourier transform infrared spectroscopy
- USP:
-
United States pharmacopeia
- PPI:
-
Polymeric precipitation inhibitor
- PVP:
-
Polyvinylpyrrolidone
- HPMC:
-
Hydroxypropylmethyl cellulose
- MC:
-
Medium chain
- LC:
-
Long chain
- SNEDDS:
-
Self nano-emulsifying drug delivery system
- SMEDDS:
-
Self micro-emulsifying drug delivery system
- SAXS:
-
Small-angle x-ray scattering
- NMR:
-
Nuclear magnetic resonance
REFERENCES
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Chen S, Dudhedia MS, Wang Z, Darrington RT, Tamblyn T, Smoliga JA, et al. Drug-excipient complexation in lipid based delivery systems: an investigation of the Tipranavir-1,3-dioctanolyglycerol complex. J Pharm Sci. 2009;98(5):1732–43.
Ku MS. Use of the biopharmaceutical classification system in early drug development. AAPS J. 2008;10(1):208–12.
Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58(2):265–78.
Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013;2013:848043.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Bevernage J, Brouwers J, Annaert P, Augustijns P. Drug precipitation-permeation interplay: supersaturation in an absorptive environment. Eur J Pharm Biopharm. 2012;82(2):424–8.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
MacGregor KJ, Embleton JK, Lacy JE, Perry EA, Solomon LJ, Seager H, et al. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv Drug Deliv Rev. 1997;25(1):33–46.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321(1–2):1–11.
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10.
Grohganz H, Priemel PA, Lobmann K, Nielsen LH, Laitinen R, Mullertz A, et al. Refining stability and dissolution rate of amorphous drug formulations. Expert Opin Drug Deliv. 2014;11(6):977–89.
Mohsin K, Long MA, Pouton CW. Design of lipid-based formulations for oral administration of poorly water-soluble drugs: precipitation of drug after dispersion of formulations in aqueous solution. J Pharm Sci. 2009;98(10):3582–95.
Mu H, Holm R, Mullertz A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 2013;453(1):215–24.
O’Driscoll CM, Griffin BT. Biopharmaceutical challenges associated with drugs with low aqueous solubility--the potential impact of lipid-based formulations. Adv Drug Deliv Rev. 2008;60(6):617–24.
Porter CJ, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60(6):673–91.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11 Suppl 2:S93–8.
Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJ. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30(12):2976–92.
Liao TH, Hamosh P, Hamosh M. Fat digestion by lingual lipase: mechanism of lipolysis in the stomach and upper small intestine. Pediatr Res. 1984;18(5):402–9.
Holm R, Mullertz A, Mu H. Bile salts and their importance for drug absorption. Int J Pharm. 2013;453(1):44–55.
Phan S, Hawley A, Mulet X, Waddington L, Prestidge CA, Boyd BJ. Structural aspects of digestion of medium chain triglycerides studied in real time using sSAXS and Cryo-TEM. Pharm Res. 2013;30(12):3088–100.
Trevaskis NL, Porter CJ, Charman WN. Bile increases intestinal lymphatic drug transport in the fasted rat. Pharm Res. 2005;22(11):1863–70.
Yeap YY, Trevaskis NL, Quach T, Tso P, Charman WN, Porter CJ. Intestinal bile secretion promotes drug absorption from lipid colloidal phases via induction of supersaturation. Mol Pharm. 2013;10(5):1874–89.
Kossena GA, Charman WN, Boyd BJ, Porter CJ. Influence of the intermediate digestion phases of common formulation lipids on the absorption of a poorly water-soluble drug. J Pharm Sci. 2005;94(3):481–92.
van Mourik ID, Thomson M, Kelly DA. Comparison of pharmacokinetics of Neoral and Sandimmune in stable pediatric liver transplant recipients. Liver Transpl Surg. 1999;5(2):107–11.
Han SF, Yao TT, Zhang XX, Gan L, Zhu C, Yu HZ, et al. Lipid-based formulations to enhance oral bioavailability of the poorly water-soluble drug anethol trithione: effects of lipid composition and formulation. Int J Pharm. 2009;379(1):18–24.
Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25(1):103–28.
Chakrabarti S, Belpaire FM. Biovailability of phenytoin in lipid containing dosage forms in rats. J Pharm Pharmacol. 1978;30(5):330–1.
Carrigan PJ, Bates TR. Biopharmaceutics of drugs administered in lipid-containing dosage forms. I. GI absorption of griseofulvin from an oil-in-water emulsion in the rat. J Pharm Sci. 1973;62(9):1476–9.
Porter CJ, Kaukonen AM, Taillardat-Bertschinger A, Boyd BJ, O’Connor JM, Edwards GA, et al. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J Pharm Sci. 2004;93(5):1110–21.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Anby MU, Williams HD, McIntosh M, Benameur H, Edwards GA, Pouton CW, et al. Lipid digestion as a trigger for supersaturation: evaluation of the impact of supersaturation stabilization on the in vitro and in vivo performance of self-emulsifying drug delivery systems. Mol Pharm. 2012;9(7):2063–79.
Arnold YE, Imanidis G, Kuentz MT. Advancing in-vitro drug precipitation testing: new process monitoring tools and a kinetic nucleation and growth model. J Pharm Pharmacol. 2011;63(3):333–41.
Warren DB, Benameur H, Porter CJ, Pouton CW. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: a mechanistic basis for utility. J Drug Target. 2010;18(10):704–31.
Lindfors L, Forssen S, Westergren J, Olsson U. Nucleation and crystal growth in supersaturated solutions of a model drug. J Colloid Interface Sci. 2008;325(2):404–13.
Bevernage J, Brouwers J, Brewster ME, Augustijns P. Evaluation of gastrointestinal drug supersaturation and precipitation: strategies and issues. Int J Pharm. 2013;453(1):25–35.
Kostewicz ES, Wunderlich M, Brauns U, Becker R, Bock T, Dressman JB. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J Pharm Pharmacol. 2004;56(1):43–51.
Stillhart C, Durr D, Kuentz M. Toward an improved understanding of the precipitation behavior of weakly basic drugs from oral lipid-based formulations. J Pharm Sci. 2014.
Yeap YY, Trevaskis NL, Porter CJ. The potential for drug supersaturation during intestinal processing of lipid-based formulations may be enhanced for basic drugs. Mol Pharm. 2013;10(7):2601–15.
Yeap YY, Trevaskis NL, Porter CJ. Lipid absorption triggers drug supersaturation at the intestinal unstirred water layer and promotes drug absorption from mixed micelles. Pharm Res. 2013;30(12):3045–58.
Kashchiev D. Forms and applications of the nucleation theorem. J Chem Phys. 2006;125(1):014502.
Horn D, Rieger J. Organic nanoparticles in the aqueous phase-theory, experiment, and use. Angew Chem Int Ed Engl. 2001;40(23):4330–61.
Rodriguez-Hornedo N, Murphy D. Significance of controlling crystallization mechanisms and kinetics in pharmaceutical systems. J Pharm Sci. 1999;88(7):651–60.
JW, M, Crystallisation. Oxford: Butterworth-Heinemann; 2001.
Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7(7):756–61.
Gargouri Y, Moreau H, Verger R. Gastric lipases: biochemical and physiological studies. Biochim Biophys Acta. 1989;1006(3):255–71.
Zangenberg NH, Mullertz A, Kristensen HG, Hovgaard L. A dynamic in vitro lipolysis model. I. Controlling the rate of lipolysis by continuous addition of calcium. Eur J Pharm Sci. 2001;14(2):115–22.
Sek L, Porter CJ, Kaukonen AM, Charman WN. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J Pharm Pharmacol. 2002;54(1):29–41.
Devraj R, Williams HD, Warren DB, Mullertz A, Porter CJ, Pouton CW. In vitro digestion testing of lipid-based delivery systems: calcium ions combine with fatty acids liberated from triglyceride rich lipid solutions to form soaps and reduce the solubilization capacity of colloidal digestion products. Int J Pharm. 2013;441(1–2):323–33.
Shono Y, Jantratid E, Dressman JB. Precipitation in the small intestine may play a more important role in the in vivo performance of poorly soluble weak bases in the fasted state: case example nelfinavir. Eur J Pharm Biopharm. 2011;79(2):349–56.
Thomas N, Richter K, Pedersen TB, Holm R, Mullertz A, Rades T. In Vitro lipolysis data does not adequately predict the in vivo performance of lipid-based drug delivery systems containing fenofibrate. AAPS J. 2014.
Williams HD, Sassene P, Kleberg K, Bakala-N’Goma JC, Calderone M, Jannin V, et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J Pharm Sci. 2012;101(9):3360–80.
Williams HD, Anby MU, Sassene P, Kleberg K, Bakala-N’Goma JC, Calderone M, et al. Toward the establishment of standardized in vitro tests for lipid-based formulations. 2. The effect of bile salt concentration and drug loading on the performance of type I, II, IIIA, IIIB, and IV formulations during in vitro digestion. Mol Pharm. 2012;9(11):3286–300.
Williams HD, Sassene P, Kleberg K, Calderone M, Igonin A, Jule E, et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 3: understanding supersaturation versus precipitation potential during the in vitro digestion of type I, II, IIIA, IIIB and IV lipid-based formulations. Pharm Res. 2013;30(12):3059–76.
Thomas N, Holm R, Rades T, Mullertz A. Characterising lipid lipolysis and its implication in lipid-based formulation development. AAPS J. 2012;14(4):860–71.
Salentinig S, Salentinig S, Tangso KJ, Hawley A, Boyd BJ. pH-driven colloidal transformations based on the vasoactive drug nicergoline. Langmuir. 2014;30(49):14776–81.
Arnold YE, Imanidis G, Kuentz M. Study of drug concentration effects on in vitro lipolysis kinetics in medium-chain triglycerides by considering oil viscosity and surface tension. Eur J Pharm Sci. 2011;44(3):351–8.
Harris KD. Powder diffraction crystallography of molecular solids. Top Curr Chem. 2012;315:133–77.
RA, C. Chapter 2: polarized light microscopy, in pharmaceutical microscopy. 2011. p. 321p. 139 illus, 102 illus. in color.
Sassene PJ, Knopp MM, Hesselkilde JZ, Koradia V, Larsen A, Rades T, et al. Precipitation of a poorly soluble model drug during in vitro lipolysis: characterization and dissolution of the precipitate. J Pharm Sci. 2010;99(12):4982–91.
Carstensen JT, Lai TY, Prasad VK. USP dissolution IV: comparison of methods. J Pharm Sci. 1978;67(9):1303–7.
Thomas N, Holm R, Mullertz A, Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J Control Release. 2012;160(1):25–32.
Savolainen M, Kogermann K, Heinz A, Aaltonen J, Peltonen L, Strachan C, et al. Better understanding of dissolution behaviour of amorphous drugs by in situ solid-state analysis using Raman spectroscopy. Eur J Pharm Biopharm. 2009;71(1):71–9.
Verdonck E, Schaap K, Thomas LC. A discussion of the principles and applications of Modulated Temperature DSC (MTDSC). Int J Pharm. 1999;192(1):3–20.
Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech. 2010;21(4):167–93.
Patel DD, Anderson BD. Effect of precipitation inhibitors on indomethacin supersaturation maintenance: mechanisms and modeling. Mol Pharm. 2014;11(5):1489–99.
Warren DB, Bergstrom CA, Benameur H, Porter CJ, Pouton CW. Evaluation of the structural determinants of polymeric precipitation inhibitors using solvent shift methods and principle component analysis. Mol Pharm. 2013;10(8):2823–48.
DiNunzio JC, Miller DA, Yang W, McGinity JW, Williams 3rd RO. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol Pharm. 2008;5(6):968–80.
Rupprecht H, Ziller KH. Characterization of the crystallization behavior of poorly soluble drugs in suspensions. Pharmazie. 1981;36(4):298.
Augustijns P, Brewster ME. Supersaturating drug delivery systems: fast is not necessarily good enough. J Pharm Sci. 2012;101(1):7–9.
Machefer S, Huddar MM, Schnitzlein K. Effect of polymer admixtures on the growth habit of ionic crystals. Study on crystal growth kinetics of potassium dihydrogen phosphate in water/polyol mixtures. J Cryst Growth. 2008;310(24):5347–56.
Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212(2):213–21.
Gao P, Akrami A, Alvarez F, Hu J, Li L, Ma C, et al. Characterization and optimization of AMG 517 Supersaturatable Self-Emulsifying Drug Delivery System (S-SEDDS) for improved oral absorption. J Pharm Sci. 2009;98(2):516–28.
Psachoulias D, Vertzoni M, Goumas K, Kalioras V, Beato S, Butler J, et al. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm Res. 2011;28(12):3145–58.
Carlert S, Akesson P, Jerndal G, Lindfors L, Lennernas H. Abrahamsson, B, In vivo dog intestinal precipitation of mebendazole: a basic BCS class II drug. Mol Pharm. 2012;9(10):2903–11.
Psachoulias D, Vertzoni M, Butler J, Busby D, Symillides M, Dressman J, et al. An in vitro methodology for forecasting luminal concentrations and precipitation of highly permeable lipophilic weak bases in the fasted upper small intestine. Pharm Res. 2012;29(12):3486–98.
Larsen AT, Sassene P, Mullertz A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int J Pharm. 2011;417(1–2):245–55.
Thomas N, Holm R, Garmer M, Karlsson JJ, Mullertz A, Rades T. Supersaturated self-nanoemulsifying drug delivery systems (Super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. AAPS J. 2013;15(1):219–27.
Boyd BJ, Khoo SM, Whittaker DV, Davey G, Porter CJ. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int J Pharm. 2007;340(1–2):52–60.
Kaukonen AM, Boyd BJ, Porter CJ, Charman WN. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm Res. 2004;21(2):245–53.
Kossena GA, Charman WN, Boyd BJ, Porter CJ. A novel cubic phase of medium chain lipid origin for the delivery of poorly water soluble drugs. J Control Release. 2004;99(2):217–29.
Larsen AT, Ohlsson AG, Polentarutti B, Barker RA, Phillips AR, Abu-Rmaileh R, et al. Oral bioavailability of cinnarizine in dogs: relation to SNEDDS droplet size, drug solubility and in vitro precipitation. Eur J Pharm Sci. 2013;48(1–2):339–50.
Hsieh YL, Ilevbare GA, Van Eerdenbrugh B, Box KJ, Sanchez-Felix MV, Taylor LS. pH-Induced precipitation behavior of weakly basic compounds: determination of extent and duration of supersaturation using potentiometric titration and correlation to solid state properties. Pharm Res. 2012;29(10):2738–53.
Hsieh YL, Box K, Taylor LS. Assessing the impact of polymers on the pH-induced precipitation behavior of poorly water soluble compounds using synchrotron wide angle x-ray scattering. J Pharm Sci. 2014;103(9):2724–35.
Lobmann K, Laitinen R, Grohganz H, Gordon KC, Strachan C, Rades T. Coamorphous drug systems: enhanced physical stability and dissolution rate of indomethacin and naproxen. Mol Pharm. 2011;8(5):1919–28.
Willart JF, Descamps M. Solid state amorphization of pharmaceuticals. Mol Pharm. 2008;5(6):905–20.
Miroshnyk I, Mirza S, Sandlert N. Pharmaceutical co-crystals-an opportunity for drug product enhancement. Expert Opin Drug Deliv. 2009;6(4):333–41.
Stillhart C, Imanidis G, Kuentz M. Insights into drug precipitation kinetics during in vitro digestion of a lipid-based drug delivery system using in-line raman spectroscopy and mathematical modeling. Pharm Res. 2013;30(12):3114–30.
Warren DB, Anby M, Hawley U, Boyd A, B. J. Real time evolution of liquid crystalline nanostructure during the digestion of formulation lipids using synchrotron small-angle X-ray scattering. Langmuir. 2011;27(15): 9528–34.
Salentinig S, Phan S, Khan J, Hawley A, Boyd BJ. Formation of highly organized nanostructures during the digestion of milk. ACS Nano. 2013;7(12):10904–11.
Phan S, Salentinig S, Prestidge CA, Boyd BJ. Self-assembled structures formed during lipid digestion: characterization and implications for oral lipid-based drug delivery systems. Drug Deliv Transl Res. 2014;4(3):275–94.
Khan J, Hawley A. Rades T, Boyd BJ. In situ lipolysis and synchrotron small-angle x-ray scattering for the direct determination of the precipitation and solid-state form of a poorly water-soluble drug during digestion of a lipid-based formulation. J Pharm Sci. 2015.
Ueda H, Ida Y, Kadota K, Tozuka Y. Raman mapping for kinetic analysis of crystallization of amorphous drug based on distributional images. Int J Pharm. 2014;462(1–2):115–22.
ACKNOWLEDGMENTS AND DISCLOSURES
This manuscript was supported by the Australian Research Council through the Discovery Projects scheme (DP120104032). B.J.B. holds an ARC Future Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, J., Rades, T. & Boyd, B. The Precipitation Behavior of Poorly Water-Soluble Drugs with an Emphasis on the Digestion of Lipid Based Formulations. Pharm Res 33, 548–562 (2016). https://doi.org/10.1007/s11095-015-1829-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1829-5