Abstract
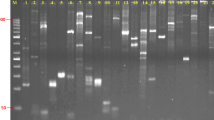
Molecular studies in sugarcane are relatively limited because of the complex genetic structure, long life cycle and non availability of reliable sugarcane specific molecular markers. The use of DNA markers for the genetic analysis and manipulation of desirable agronomic traits has become an increasingly useful tool in sugarcane breeding. The objective of the present study was to evaluate the polymorphic potential of sugarcane microsatellite markers developed at National Research Centre on Plant Biotechnology, IARI, New Delhi in high and low sugar lines. These microsatellites markers were screened and validated for their polymorphic, genetic diversity, cross-transferability and comparative linkage mapping potential in high and low sugar bulk of two segregating progenies and 20 each, cultivated high and low sugar commercial varieties. One hundred sixty eight (28%) of the microsatellite markers were found to be highly robust and polymorphic with PIC values ranging from 0.51% to 0.84%. Forty three (0.26 %) markers contained dinucleotide repeats, fifty seven (0.34 %) markers contained trinucleotide repeats, twenty five (0.15 %) and forty three (0.26 %) markers contained composite repeats. The number of observed allele ranged from 2 to 11, with an average of five alleles detected per locus. A total of 977 polymorphic DNA bands were identified, with their fragment size ranging from 20 to 1380 bp. These microsatellite markers are an ideal resource for use in managing sugarcane germplasm, trait mapping and marker assisted breeding strategies. The wide cross — species transportability of these markers may extend their value to research involving other Saccharum species complex.
Similar content being viewed by others
References
Beese P, McIntyre CL (1996) Molecular markers: Useful tools for sugarcane breeders. In Sugarcane: research towards efficient and sustainable production. J. R. Wilson, D. M. Hogarth, J. A. Campbell and A. L. Garside (Eds), pp 57–58.
Beese P, McIntyre CL, Berding N (1996) Ribosomal DNA varieties in Erianthus, a wild sugarcane relative (Andropogoneae saccharinae). Theor. Appl. Genet 92: 733–743.
Beese P, Taylor G, Carroll B, Berding N, Burner DM, McIntyre CL (1998) Assessing genetic diversity in a sugarcane germplasm collection using an automated AFLP analysis. Genetica, 104:143–153.
Burner DM, Pan Y-B, Webster RD (1997) Genetic diversity of New World and Old World Sacchrum assessed by RAPD Analysis. Genet. Res. Crop Evol., 44:235–240.
Burner DM, Legendre BL (1993) Sugarcane genome amplification for the subtropics: a twenty year effort. Sugar Cane, 3: 5–10.
Burnquist WB (1991) Development and application of restriction fragment length polymorphism technology in sugarcane (Saccharum spp.) breeding. Ph. D dissertation, Cornell Univ., Ithaca, NY.
Cordeiro GM, Henry RJ (2001) Evaluation of microsatellites (Simple Sequence Repeats) as genetic markers in sugarcane. Proc. Intl. Soc. Sugar Cane Technol., 24: 627–629.
Cordeiro GM, Pan YB, Henry RJ (2003) Sugarcane microsatellites for the assessment of genetic diversity in sugarcane germplasm. Plant Sci. 165:181–189.
Cordeiro GM, Taylor GO, Henry RJ (2000) Characterization of microsatellites markers from sugarcane (Saccharum spp.), a highly polyploid species, Plant Sci 155: 161–168.
D’Hont A, Lu Y H, Feldmann P, Glaszmann JC (1993) Cytoplasmic diversity in sugar cane revealed by heterologous probes. Sugar Cane 1:12–15.
D’Hont A, Rao PS, Feldmann P, Grivet L, Islam FN, Taylor P, Glaszmann JC (1995) Identification and characterization of sugarcane intergeneric hybrids, Saccharum officinarum & Erianthus arundinaceus, with molecular markers and DNA in situ hybridization. Theor. Appl. Genet. 91: 320–326.
Da Silva JAG (2001) Preliminaru analysis of microsatellite markers derived from sugarcane expressed sequence tags (ESTs). Genet. Mol. Biol. 24: 155–159.
Dellaporta SL, Wood J, Hicks TB (1983) A plant DNA mini preparation version II. Plant Mol. Biol. Rep. 1, pp 19–20.
Edward A, Civitello A, Hammond HA, Caskey CT (1991) Genotyping and genetic mapping with trimeric and tetrameric tendem repeats. Am.J. Human Genet 59:746–756.
Duckelman PH, Legendre BL (1982) Guide to sugarcane breeding in the template zone. USDA-ARS, ARM-S-22. New Orleans
Glaszmann JC, Noyer JL, Fautret A, Lanaud C (1990) Variation of nuclear ribosomal DNA in sugarcane. J. Genet. Breed, 44:191–198.
Glaszmann JC, Fautret A, Noyer J L, Feldmann P, Lanaud C (1989) Biochemical genetic markers in sugarcane. Theor. Appl. Genet. 78:537–543. (Saccharum spp.):Genome organization in a highly polyploid and aneuploid interspecific hybrid. Genetics 142: 987–1000.
Harvey M, Botha FC (1996) Use of PCR- based methodologies for the determination of DNA diversity between Saccharum varieties. Euphytica, 89: 257–265.
Janoo N, Forget L, Dookun A (2001) Contribution of microsatellite to sugarcane breeding program in Maurtius. Proc. Intl.Soc. Sugar Cane Technol, 24: 637–639.
Milbourne D, Meyer R, Bradshaw JE, Baird E, Bonar N, Povan J, Powell W, Waugh R (1997) Comparison of PCR-based marker system the analysis of genetic relationships in cultivated potato. Mol. Breed., 3:127-136.
Ming R, Liu SC, Lin YR, Braga D, Silva J da, van Deynze A, Wenslaff TF, Wu KK, Moore PH, Burnquist W, Sorrells ME, Irvine JE, Paterson AH (1998) Alignment of Sorghum and Saccharum Chromosomes: Comparative organization of closely related diploid and polyploid genomes. Genetics 150:1663–1682.
Pan YB, Burner DM, Legendre BL (2000) An assessment of the polygenetic relationship among sugarcane and related taxa based on the nucleotide sequence of 5S rRNA intergenic spacers. Genetica, 108: 285–295.
Pan YB, Burner DM, Legendre BL, Grisham MP, White, WH (2004) An assessment of the genetic diversity within a collection of Saccharum spontaneum with RAPD-PCR. Genet. Res Crop Evol., 51: 895–903.
Pan YB (2006) Highly polymorphic microsatellite DNA markers for sugarcane germplasm evaluation and variety identity testing. Sugar Tech, 8(4): 246–256.
Pan YB, Burner DM, Wei Q (2001) Developing species-specific DNA markers to assist in sugarcane breeding. Proc. Intl. Sugar Cane Technol. 24(II): 337–342.
Pan YB, Cordeiro GM Richard Jr, EP, Henry RJ (2003a, b) Molecular genotyping of sugarcane clones with microsatellite DNA markers. Maydica, 48: 319–329.
Pan YB, Miller JD, Schnell RJ, Richard Jr, RP, Wei Q (2003) Application of microsatellite and RAPD fingerprints in the Florida sugarcane variety program. Sugar Cane International, 19–28.
Pan YB, Burner DM, Ehrlich KC, Grisham MP, Wei Q (1997) Analysis of primer-derived, non specific amplification products in RAPDPCR. BIO Techniques, 22(6):1072–1074.
Parida SK, Kalia SK, Kaul S, Dalal V, Hemprapha G, Selvi A, Pandit A, Singh A, Gaikwad K, Sharma TR, Srivastava PS, Singh NK, Mohapatra T (2008a). Informative genomic microsatellite markers for efficient genotyping application in sugarcane. Theor. Appl. Genet. DO110.1007/S00122-008-0902-4.
Parida SK, Dalal V, Pandit A, Selvi A, Sharma TR, Srivastava PS, Singh NK, Mohapatra T (2008b). Development and use of unigene derived microsatellite markers for assessment of genetic diversity in sugarcane (Unpublished).
Piperidis G, Christopher MJ, Carroll BJ, Berding N, D’Hont A (2000) Molecular contribution to selection of intergeneric hybrids between sugarcane and the wild species Erianthus arundinaceus. Genome 43:1033–1037.
Piperidis G, Taylor GO, Smith GR, (2001) A microsatellite marker database for fingerprinting sugarcane clones. Proc. Intl. Soc Sugar Cane Technol. 24: 632–633.
Polymeropoulos M.H., H. Xiao., D.S. Rath., C.R. Merril (1991). Tetra nucleotide repeat polymorphism at the human tyrosine hydroxylase gene. Nuc. Acids Res. 19:3753
Singh RK, Singh P, Mishra P, Singh SP, Singh SB (2005) STMS markers for tagging high sugar genes in sugarcane. Sugar Tech, 7(2&3): 74–76.
Tew TL, Pan YB (2005) Molecular assessment of the fidelity of sugarcane crosses with high throughput microsatellite genotyping. J. Amer. Soc. Sugar Cane Technol., 25: 119.
Weber JL, May PE (1989) Abundant class of human DNA polymorphism which can be typed using the polymerase chain reaction. Am. J. Hum. Genet. 44:388–396.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R.K., Srivastava, S., Singh, S.P. et al. Identification of new microsatellite DNA markers for sugar and related traits in sugarcane. Sugar Tech 10, 327–333 (2008). https://doi.org/10.1007/s12355-008-0058-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-008-0058-1