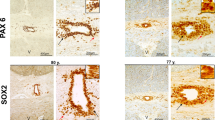
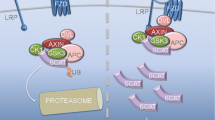
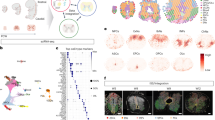
Abstract
The Wnt family of proteins plays key roles during central nervous system development and in several physiological processes during adulthood. Recently, experimental evidence has linked Wnt-related genes to regulation and maintenance of stem cells in the adult neurogenic niches. In the spinal cord, the ependymal cells surrounding the central canal form one of those niches, but little is known about their Wnt expression patterns. Using microdissection followed by TaqMan® low-density arrays, we show here that the ependymal regions of young, mature rats and adult humans express several Wnt-related genes, including ligands, conventional and non-conventional receptors, co-receptors, and soluble inhibitors. We found 13 genes shared between rats and humans, 4 exclusively expressed in rats and 9 expressed only in humans. Also, we observed a reduction with age on spontaneous proliferation of ependymal cells in rats paralleled by a decrease in the expression of Fzd1, Fzd8, and Fzd9. Our results suggest a role for Wnts in the regulation of the adult spinal cord neurogenic niche and provide new data on the specific differences in this region between humans and rodents.





Similar content being viewed by others
References
Alvarez-Buylla A, Garcia-Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22:629–634
Silva-Vargas V, Crouch EE, Doetsch F (2013) Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol 23:935–942
Bond AM, Ming GL, Song H (2015) Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 17:385–395
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J (2010) Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7:470–482
Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH et al (2000) Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 20:2218–2228
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96:25–34
Panayiotou E, Malas S (2013) Adult spinal cord ependymal layer: a promising pool of quiescent stem cells to treat spinal cord injury. Front Physiol 4:340
Qin Y, Zhang W, Yang P (2015) Current states of endogenous stem cells in adult spinal cord. J Neurosci Res 93:391–398
Sabourin JC, Ackema KB, Ohayon D, Guichet PO, Perrin FE, Garces A, Ripoll C, Charité J et al (2009) A mesenchymal-like ZEB1(+) niche harbors dorsal radial glial fibrillary acidic protein-positive stem cells in the spinal cord. Stem Cells 27:2722–2733
Shihabuddin LS, Ray J, Gage FH (1997) FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol 148:577–586
Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609
Alfaro-Cervello C, Cebrian-Silla A, Soriano-Navarro M, Garcia-Tarraga P, Matías-Guiu J, Gomez-Pinedo U, Molina Aguilar P, Alvarez-Buylla A et al (2014) The adult macaque spinal cord central canal zone contains proliferative cells and closely resembles the human. J Comp Neurol 522:1800–1817
Dromard C, Guillon H, Rigau V, Ripoll C, Sabourin JC, Perrin FE, Scamps F, Bozza S et al (2008) Adult human spinal cord harbors neural precursor cells that generate neurons and glial cells in vitro. J Neurosci Res 86:1916–1926
Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Florensa-Vila J, Ferrer I, Grassner L, Molina-Holgado E (2015) The ependymal region of the adult human spinal cord differs from other species and shows ependymoma-like features. Brain 138:1583–1597
Hugnot JP, Franzen R (2011) The spinal cord ependymal region: a stem cell niche in the caudal central nervous system. Front Biosci (Landmark Ed) 16:1044–1059
Kasantikul V, Netsky MG, James AE Jr (1979) Relation of age and cerebral ventricle size to central canal in man. Morphological analysis. J Neurosurg 51:85–93
Milhorat TH, Kotzen RM, AP A (1994) Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg 80:716–722
Yasui K, Hashizume Y, Yoshida M, Kameyama T, Sobue G (1999) Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol 97:253–259
Mothe AJ, Zahir T, Santaguida C, Cook D, Tator CH (2011) Neural stem/progenitor cells from the adult human spinal cord are multipotent and self-renewing and differentiate after transplantation. PLoS One 6:e27079
Alfaro-Cervello C, Soriano-Navarro M, Mirzadeh Z, Alvarez-Buylla A, Garcia-Verdugo JM (2012) Biciliated ependymal cell proliferation contributes to spinal cord growth. J Comp Neurol 520:3528–3552
Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Sierra-Palomares Y, Molina-Holgado E (2013) A cell population that strongly expresses the CB1 cannabinoid receptor in the ependyma of the rat spinal cord. J Comp Neurol 521:233–251
Hamilton LK, Truong MK, Bednarczyk MR, Aumont A, Fernandes KJ (2009) Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience 164:1044–1056
Kempermann G (2015) Activity dependency and aging in the regulation of adult neurogenesis. Cold Spring Harb Perspect Biol 7
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033
Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC (2006) The aging neurogenic subventricular zone. Aging Cell 5:139–152
Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116:769–778
Morrison SJ, Spradling AC (2008) Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132:598–611
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Ciani L, Salinas PC (2005) WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci 6:351–362
Ille F, Sommer L (2005) Wnt signaling: multiple functions in neural development. Cell Mol Life Sci 62:1100–1108
Lyuksyutova AI et al (2003) Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302:1984–1988
Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129:2087–2098
Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ (2011) Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J Neurosci 31:1676–1687
Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S (2002) Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev 16:548–553
Cerpa W, Ramos-Fernandez E, Inestrosa NC (2016) Modulation of the NMDA receptor through secreted soluble factors. Mol Neurobiol 53:299–309
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, Zhao C, Du H et al (2012) Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun 420:397–403
Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ (2011) Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One 6:e27000
Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S (2010) Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J 29:2433–2445
Gonzalez-Fernandez C, Fernandez-Martos CM, Shields S, Arenas E, Rodriguez FJ (2013) Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma 31:565–581
Gonzalez-Fernandez C, Mancuso R, Del Valle J, Navarro X, Rodriguez FJ (2016) Wnt signaling alteration in the spinal cord of amyotrophic lateral sclerosis transgenic mice: special focus on frizzled-5 cellular expression pattern. PLoS One 11:e0155867
Gonzalez P, Fernandez-Martos CM, Arenas E, Rodriguez FJ (2013) The Ryk receptor is expressed in glial and fibronectin-expressing cells after spinal cord injury. J Neurotrauma 30:806–817
Gonzalez P, Fernandez-Martos CM, Gonzalez-Fernandez C, Arenas E, Rodriguez FJ (2012) Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS One 7:e50793
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11:77–86
Inestrosa NC, Toledo EM (2008) The role of Wnt signaling in neuronal dysfunction in Alzheimer’s disease. Mol Neurodegener 3:9
Lambert C, Cisternas P, Inestrosa NC (2016) Role of Wnt signaling in central nervous system injury. Mol Neurobiol 53:2297–2311
Li X, Yingjun G, Yanchun C, Caixia Z, Caixing S, Fenghua Z, Li Y, Juan J et al (2013) Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int J Clin Exp Pathol 6:1245–1260
Michaelidis TM, Lie DC (2008) Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res 331:193–210
Parish CL, Gonçalo CB, Rawal N, Tonnesen J, Sorensen AT, Salto C, Kokaia M, Lindvall O et al (2008) Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest 118:149–160
Wang S, Guan Y, Chen Y, Li X, Zhang C, Yu L, Zhou F, Wang X (2013) Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnol Lett 35:1199–1207
Yu L, Guan Y, Wu X, Chen Y, Liu Z, Du H, Wang X (2013) Wnt signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem Res 38:1904–1913
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477
Montcouquiol M, Crenshaw EB III, Kelley MW (2006) Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci 29:363–386
Widelitz R (2005) Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors 23:111–116
Buechling T, Boutros M (2011) Wnt signaling signaling at and above the receptor level. Curr Top Dev Biol 97:21–53
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13:767–779
Schulte G, Bryja V (2007) The frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci 28:518–525
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci 121:737–746
Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149:1192–1205
Lange C, Mix E, Rateitschak K, Rolfs A (2006) Wnt signal pathways and neural stem cell differentiation. Neurodegener Dis 3:76–86
Hu Z, Zimmermann BG, Zhou H, Wang J, Henson BS, Yu W, Elashoff D, Krupp G et al (2008) Exon-level expression profiling: a comprehensive transcriptome analysis of oral fluids. Clin Chem 54:824–832
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Team RC (2015) R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013 Document freely available on the internet at: http://www r-project org
Conover JC, Shook BA (2011) Aging of the subventricular zone neural stem cell niche. Aging Dis 2:49–63
Mardones MD et al (2016) Frizzled-1 receptor regulates adult hippocampal neurogenesis. Mol Brain 9:29
Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C, Haug JS, Peng L et al (2012) Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150:351–365
Zhao C, Pleasure SJ (2005) Frizzled9 protein is regionally expressed in the developing medial cortical wall and the cells derived from this region. Brain Res Dev Brain Res 157:93–97
Moya N, Cutts J, Gaasterland T, Willert K, Brafman DA (2014) Endogenous WNT signaling regulates hPSC-derived neural progenitor cell heterogeneity and specifies their regional identity. Stem Cell Reports 3:1015–1028
Nusse R (2008) Wnt signaling and stem cell control. Cell Res 18:523–527
Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434:843–850
Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH (2009) Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 27:1130–1141
Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J (2008) Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 6:e182
Capilla-Gonzalez V, Cebrian-Silla A, Guerrero-Cazares H, Garcia-Verdugo JM, Quinones-Hinojosa A (2014) Age-related changes in astrocytic and ependymal cells of the subventricular zone. Glia 62:790–803
Ngwenya LB, Heyworth NC, Shwe Y, Moore TL, Rosene DL (2015) Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci 9:102
Israsena N, Hu M, Fu W, Kan L, Kessler JA (2004) The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol 268:220–231
Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L et al (2007) Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 25:2827–2836
Cajanek L, Ribeiro D, Liste I, Parish CL, Bryja V, Arenas E (2009) Wnt/beta-catenin signaling blockade promotes neuronal induction and dopaminergic differentiation in embryonic stem cells. Stem Cells 27:2917–2927
Zhang L, Yang X, Yang S, Zhang J (2011) The Wnt /beta-catenin signaling pathway in the adult neurogenesis. Eur J Neurosci 33:1–8
David MD, Canti C, Herreros J (2010) Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J Neurosci Res 88:3011–3023
Lie DC et al (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437:1370–1375
Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T (2011) Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J 25:3570–3582
He Y, Zhang PZ, Sun D, Mi WJ, Zhang XY, Cui Y, Jiang XW, Mao XB et al (2014) Wnt1 from cochlear schwann cells enhances neuronal differentiation of transplanted neural stem cells in a rat spiral ganglion neuron degeneration model. Cell Transplant 23:747–760
Yu JM, Kim JH, Song GS, Jung JS (2006) Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol Cell Biochem 288:17–28
Elizalde C, Campa VM, Caro M, Schlangen K, Aransay AM, Vivanco M, Kypta RM (2011) Distinct roles for Wnt-4 and Wnt-11 during retinoic acid-induced neuronal differentiation. Stem Cells 29:141–153
Lyu J, Yamamoto V, Lu W (2008) Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell 15:773–780
Kannan S, Bonaguidi MA, Kitabatake Y, Su J, Song J, Kang E, Jun H, Zhong C et al (2016) Systems genetics analysis of a recombinant inbred mouse cell culture panel reveals Wnt pathway member Lrp6 as a regulator of adult hippocampal precursor cell proliferation. Stem Cells 34:674–684
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634
Zhu Y, Demidov ON, Goh AM, Virshup DM, Lane DP, Bulavin DV (2014) Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. J Clin Invest 124:3263–3273
Kele J et al (2012) SFRP1 and SFRP2 dose-dependently regulate midbrain dopamine neuron development in vivo and in embryonic stem cells. Stem Cells 30:865–875
Jang MH et al (2013) Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12:215–223
Sun J, Bonaguidi MA, Jun H, Guo JU, Sun GJ, Will B, Yang Z, Jang MH et al (2015) A septo-temporal molecular gradient of sfrp3 in the dentate gyrus differentially regulates quiescent adult hippocampal neural stem cell activation. Mol Brain 8:52
Palm T, Figarella-Branger D, Chapon F, Lacroix C, Gray F, Scaravilli F, Ellison DW, Salmon I et al (2009) Expression profiling of ependymomas unravels localization and tumor grade-specific tumorigenesis. Cancer 115:3955–3968
Cantilena S, Pastorino F, Pezzolo A, Chayka O, Pistoia V, Ponzoni M, Sala A (2011) Frizzled receptor 6 marks rare, highly tumourigenic stem-like cells in mouse and human neuroblastomas. Oncotarget 2:976–983
Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao S, Gaasterland T et al (2014) The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proc Natl Acad Sci U S A 111:1409–1414
Melchior K, Andaur GA, Varas-Godoy M, Henriquez JF, Salech F, Behrens MI, Couve A, Inestrosa NC et al (2008) The WNT receptor FZD7 contributes to self-renewal signaling of human embryonic stem cells. Biol Chem 389:897–903
Endo M, Doi R, Nishita M, Minami Y (2012) Ror family receptor tyrosine kinases regulate the maintenance of neural progenitor cells in the developing neocortex. J Cell Sci 125:2017–2029
Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T et al (2013) Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 12:204–214
Verani R, Cappuccio I, Spinsanti P, Gradini R, Caruso A, Magnotti MC, Motolese M, Nicoletti F et al (2007) Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem 100:242–250
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53
Anastas JN, Moon RT (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13:11–26
Holland JD, Klaus A, Garratt AN, Birchmeier W (2013) Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 25:254–264
Acknowledgments
We are grateful to Concepción Sanchez-Caro and Uyen Le for technical help. We also thank Dr. Pau Gonzalez for his expert technical support. This work was funded by Wings For Life Foundation (Salzburg, Austria) and Fundacion Mutua Madrileña (Madrid, Spain) and by grants obtained from the Fondo de Investigación Sanitaria (FIS) (Grant PI12-2895, co-funded by Fondo Europeo de Desarrollo Regional [FEDER]). Laboratory of Neuroinflammation is also supported by Instituto de Salud Carlos III (Ministry of Economy and Competitiveness of Spain).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Use of Animals in Neuroscience Research published by the Society for Neuroscience and the European Union guidelines (Council Directive 2010/63/UE). Experimental procedures were approved by our Local Ethical Committee for Animal Research (CEEA).
Donation always included a written informed consent from donors while alive or from their families after death. Data from donors and handling of samples were carried out after approval by the Clinical Research Ethical Committee (CEIC) in Toledo (Spain), in accordance with the Spanish law and International Guidelines (LOPD 15/1999; RD 1720/2007; Helsinki Declaration, 2008).
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gonzalez-Fernandez, C., Arevalo-Martin, A., Paniagua-Torija, B. et al. Wnts Are Expressed in the Ependymal Region of the Adult Spinal Cord. Mol Neurobiol 54, 6342–6355 (2017). https://doi.org/10.1007/s12035-016-0132-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0132-8