Abstract
Background
Component alignment can influence implant longevity as well as perhaps pain and function after unicompartmental knee arthroplasty (UKA), but correct alignment is not consistently achieved. To increase the likelihood that good alignment will be achieved during surgery, smart tools such as robotics or patient-specific instrumentation (PSI) have been introduced.
Questions/purposes
We hypothesized that UKA performed with PSI would result in improved level gait as ascertained with three-dimensional analysis, implant positioning, and patient-reported outcomes measured by a validated scoring system when compared with conventional instrumentation 3 months and 1 year after surgery.
Methods
We randomized 60 patients into two groups using either the PSI technique or a conventional technique. All patients were operated on using the same technique and the same cemented metal-backed implant. Mean age of the patients was 63 ± 4 years (range, 54–72 years) and mean body mass index was 28 ± 3 kg/m2. Patients were evaluated preoperatively, at 3 months, and 1 year after surgery by an independent observer blind to the type of technique. Gait parameters were assessed with three-dimensional analysis during level walking preoperatively and at 1 year, frontal and sagittal position of the implant was evaluated on full-length radiographs at 3 months, and subjective functional outcome and quality of life using routine questionnaires (SF-12, new Knee Society Score [KSS], Knee Injury and Osteoarthritis Outcome Score) at 3 months and 1 year. This study had 80% power to detect a 15% difference in walking speed at the p < 0.05 level.
Results
One year after surgery, there were no differences between the two groups in the analyzed gait spatiotemporal parameters, respectively, for PSI UKA and conventional UKA : double limb support 31% (25%–54%) versus 30% (23%–56%; p = 0.67) and walking speed (1.59 m/s [0.86–1.87 m/s] versus 1.57 m/s [0.71–1.96 m/s]; p = 0.41). No difference was observed between the two groups in terms of lower limb alignment (PSI group 178° ± 3°, conventional group 178° ± 4°; p = 0.24) or implant positioning on mediolateral and anteroposterior radiographs. There were no differences in the functional score between the PSI and conventional TKA groups at 3 months and 1 year after surgery: KSS objective knee scores (PSI: 85 ± 8 points at 3 months, 87 ± 5 points at 1 year and conventional instrumentation: 82 ± 8 points at 3 months 83 ± 6 points at 1 year; p = 0.10) and functional activity scores were similar in both group (PSI: 71 ± 12 points at 3 months and 74 ± 7 points at 1 year versus conventional group: 73 ± 11 points at 3 months and 75 ± 6 at 1 year; p = 0.9).
Conclusions
Our observations suggest that PSI may confer small, if any, advantage in alignment, pain, or function after UKA. This argument can therefore not be used to justify the extra cost and uncertainty related to this technique.
Level of Evidence
Level I, therapeutic study.
Similar content being viewed by others
Introduction
Unicompartimental knee arthroplasty (UKA) can be a challenging procedure, and correct implant positioning is crucial to optimize postoperative function and implant survivorship [24]. Several studies have demonstrated a direct relationship among accurate implant positioning, survivorship, and functional postoperative outcomes [8, 11, 15]. Results from registries showed that the rate of revision increased when a surgeon performed fewer than 23 UKAs per year [5, 18, 24]. To improve implant positioning in UKA, computer-assisted surgery and then robotics have been developed with potential advantages in terms of implant positioning but with important drawbacks such as the cost and the time of surgery [12]. After the development of patient-specific instrumentation (PSI) in TKA, PSI has been developed for UKA with the goal of improving implant positioning and potentially functional outcomes. An MRI-based protocol is used to create the knee model and to develop the corresponding cutting blocks, which guide frontal and the sagittal cuts on the tibia as well as the distal femoral cut [10].
However, studies differ as to whether PSI for UKA results in improved alignment [7, 14] and there are few data demonstrating whether any differences achieved in alignment will translate into clinically relevant differences that a patient might perceive [15]. To our knowledge, there are no randomized clinical trials on this important topic.
We hypothesized that UKA performed with PSI would improve implant positioning, patient-reported outcomes, and gait compared with a conventional technique. Therefore, we aimed to specifically compare in a randomized controlled study (1) radiological results based on the frontal and sagittal alignment of the implants on conventional radiographs; (2) functional outcomes using the new Knee Society Score (KSS) [19], the Knee Injury and Osteoarthritis Outcome Score (KOOS), and the SF-12 at 3 months and 1 year; and (3) three-dimensional (3-D) gait parameters at 1 year of patients operated on for a UKA with PSI versus patients operated on with standard instrumentation.
Patients and Methods
In this randomized controlled trial, 60 patients (30 in each group) undergoing unilateral primary medial UKA at our institution between January and April 2013 performed by the two senior authors (J-NA and SP together 200 medial UKA performed in 2013) were recruited.
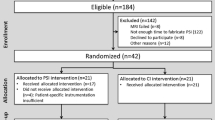
The inclusion criteria were (1) isolated symptomatic medial femorotibial knee arthritis [2]; (2) with varus deformity; (3) age between 50 and 85 years; and (4) acceptance of a new technology protocol (including delay between MRI and surgery). Exclusion criteria were (1) ROM below 0° to 100° (extension to flexion); (2) unstable knees in frontal and/or sagittal planes; (3) a personal history of trauma, sepsis, tumor, inflammatory or skeletal disease (that could influence gait parameters); (4) previous lower limb joint (ankle to hip) surgery that could lead to an artifact effect on imaging; and (5) any contraindication to MRI. All patients who met the criteria were invited to participate; 60 patients agreed to participate representing 86% of the 69 patients meeting the named criteria during the inclusion period. No patients were lost to followup (Fig. 1). Institutional review board approval was obtained and all patients provided informed consent.
Patients were randomized to either the PSI or conventional instrumentation group by the hospital’s informatics department using a previously described systematic sampling method [1]. No differences in terms of anthropometric and radiological (preoperative) parameters were found in the two groups (Table 1). For the patients allocated to the PSI group, MRIs were obtained after randomization. All patients had their MRI completed on the same machine (Philips™ Intera 1.5 Tesla; Koninklijke Philips NV, Best, The Netherlands) in the hospital’s department of radiology using a standardized protocol validated by the protocol manufacturer (Materialise™, Leuven, Belgium). After segmentation, the engineers planned the UKA and submitted the plan to the surgeon. Based on the clinical examination and standing full-length hip-to-ankle radiographs, the plan was scrutinized and modified by the surgeon to set the appropriate depth of the distal femoral and tibial cuts, flexion of the femoral implant, and slope of the tibial plateau. The aim was to obtain a 3-mm cut based on the level of the lowest point on the tibial plateau with an angle of 90° according to the mechanical axis of the tibia and with a 3° posterior slope. On the femoral side, we were aiming for a 9-mm cut perpendicular to the mechanical axis of the femur.
The surgical exposure through a medial parapatellar arthrotomy was identical for both groups. For patients randomized to the PSI group, after exposure of the knee, the PSI jigs were carefully positioned over previously cleaned and dried articular surfaces, ensuring an accurate fit. Subsequently, guided by the PSI jig, drill holes and pins were placed in the cartilage surfaces, which then determined the orientation of standard cutting guides.
For patients randomized to the conventional instrumentation group, a traditional extramedullary guide was used for the tibial cut. The remaining procedure in both groups was completed as per our standard protocol [3, 4]. In both groups patients received the identical cemented metal-backed fixed-bearing medial unicompartmental prosthesis (ZUK™; Zimmer, Warsaw, IN, USA) [4]. In both groups, no tourniquet was used and patients received identical postoperative pain and blood management protocols. In addition, the same postoperative rehabilitation protocol was used for all patients.
In the PSI group, three intraoperative modifications after performing the cuts with the PSI jigs were performed (of 30 in this group; 10%). In these patients, the surgeon judged intraoperatively that these knees required an additional tibial resection of 2 mm.
Gait parameters were analyzed 1 year after surgery in our institutional gait laboratory. The laboratory is fitted with the 3-D Vicon™ system (Viton, Denver, CO, USA), six cameras, and two AMTI™ force platforms (Advanced Mechanical Technology Inc, Watertown, MA, USA) to calculate spatiotemporal and kinematic parameters [16, 21, 23].
In each group, the radiographic analysis included pre- and postoperative full-length standing radiographs, a mediolateral view of the knee, and a skyline view at 30° of flexion. For hip-knee angle, the radiological assessment was carried out by two independent observers (MO, AL) according to a previously described method [9] on full-leg weightbearing radiographs, AP and lateral views, and on skyline patellar views. The mechanical axis of the lower limbs was measured pre- and postoperatively based on the hip-knee angle evaluation and Kennedy and Wright classification [13], the frontal femoral and tibial component angle on mediolateral and AP views as well as sagittal femoral component angle and tibial slope.
Subjective and objective functional results were analyzed preoperatively, 3 months, and 2 year postoperatively using the (1) KSS; (2) KOOS; and (3) SF-12 [17, 19, 20].
Statistical Methods
The a priori sample size calculation assumed that a difference of walking speed of 0.2 m/sec was clinically relevant [23]. Assuming the variability of this value (comfortable walking speed after UKA 1.38 ± 0.2 m/s [23]), a power of 90%, and a significance level of 5%, the required sample size was 22 patients in each group.
Data that are descriptive statistics are presented as mean ± SD. Statistical analysis was performed with SPSS™ 12.0 (IBM Corporation, Somers, NY, USA). Student’s paired t-tests were used for intragroup comparison. Two-sample t-tests were used for intergroup comparisons, and multiple analyses of variance were used for multiple comparisons (functional score analysis: preoperative/3 months/1 year).
All patients provided written consent, and the study received authorization from our local ethics committee.
Results
Gait Analysis
One year after surgery, there were no differences between the two groups in the analyzed gait spatiotemporal parameters with, respectively, PSI UKA and conventional UKA: double limb support 31% (minimum-maximum, 25%–54%) versus 30% (23%–56%; p = 0.67), single limb support 69% (36%–75%) versus 70% (44%–77%; p = 0.59), and walking speed 1.59 (0.86–1.87 m/s) versus 1.57 m/s (0.71–1.96 m/s; p = 0.41). No difference was also found between groups regarding kinematic parameters with, respectively, PSI UKA and conventional UKA knee varus angle 1.8° (−2° to –3°) versus 1.3° (−1° to 2°; p = 0.39), knee valgus angle −2° (−4° to 4°) versus −2.4° (−5° to 3°; p = 0.46), knee varus moment 0.5 Nm/kg (0.3–0.8 Nm/kg) versus 0.5 Nm/kg (0.2–0.7 Nm/kg; p = 0.71), and knee valgus moment 0 Nm/kg (−0.1 to 0.2 Nm/kg) versus −0.1 Nm/kg (−0.2 to 0.3 Nm/kg; p = 0.59) (Table 2).
Radiological Analysis
On the weightbearing full-length radiographs at 3 months, no hip-knee angle difference was observed between the two groups (PSI group 178° ± 3°, conventional group 178° ± 4°; p = 0.24). No difference was observed concerning the frontal and sagittal position of the implants on the mediolateral and AP radiographs (Table 2) with, respectively, PSI and conventional femoral frontal component angle: 89° ± 2° versus 90° ± 2° (p = 0.16), tibial frontal component angle: 89° ± 2° for both groups (p = 0.31), femoral sagittal component angle: 100° ± 2 versus 97° ± 2° (p = 0.29), and tibial slope: 4° ± 2° versus 5° ± 3° (p = 0.23) (Table 3).
Patient-reported Outcome Measures
There were no differences in the functional score between the PSI and conventional TKA groups at 3 months and 1 year after surgery; for the KSS score, we found no difference between groups at 3 months and 1 year for objective knee scores (PSI : 85 ± 8 points at 3 months, 87 ± 5 points at 1 year and conventional instrumentation: 82 ± 8 points at 3 months 83 ± 6 points at 1 year; p = 0.10) or satisfaction scores (PSI: 32 ± 5 points at 3 months, 31 ± 4 points at 1 year and conventional instrumentation: 33 ± 7 points at 3 months and 33 ± 4 points at 1 year; p = 0.08). Expectation scores were also not different at 3 months (PSI: 12 ± 3 points, conventional: 13 ± 2 points) and 1 year (PSI: 12 ± 3 points, conventional: 12 ± 2 points; p = 0.1), and functional activity scores were similar in both group (PSI: 71 ± 12 points at 3 months and 74 ± 7 points at 1 year versus conventional group: 73 ± 11 points at 3 months and 75 ± 6 at 1 year; p = 0.9). No statistical difference was found for all the KOOS subscores at 3 months or 1 year (Fig. 2). Finally, physical SF-12 subscales at 3 months (PSI: 55 ± 7 points, conventional instrumentation: 55 ± 7 points) and 1 year (PSI: 78 ± 11 points and conventional instrumentation: 81 ± 12) and mental SF-12 subscales at 3 months (PSI: 58 ± 8 points, conventional instrumentation 58 ± 10 points) and 1 year (PSI: 63 ± 9 points and conventional instrumentation: 62 ± 9 points) were not different (respectively, all p > 0.1) in both groups (Tables 4–6).
Discussion
Component alignment can influence implant longevity as well as perhaps pain and function after UKA [11, 15], but correct alignment is not consistently achieved. To increase the likelihood that good alignment will be achieved during surgery, smart tools such as robotics or PSI have been introduced [10]. However, very limited scientific data are available concerning PSI UKA in the literature. Studies have disagreed about whether PSI improves alignment in patients undergoing UKA [16, 21], but to our knowledge, no study has specifically evaluated whether PSI will result in better functional outcomes compared with traditional surgical approaches. We hypothesized that UKA performed with PSI would improve implant positioning, patient-reported outcomes, and gait compared with the conventional technique. In this randomized clinical trial comparing PSI versus conventional instrumented UKA, we found no benefit in any radiological or functional outcomes and no difference in gait parameters when PSI was used.
There are limitations to the current study. First, the sample size is small with 30 patients per group. However, the preoperative power analysis, similar nature of the two populations preoperatively, and randomized clinical study design help mitigate this issue. In addition, we have only focused on the first year postoperatively, and the results cannot be extrapolated to longer-term followup. However, it was our intention to focus on the immediate postoperative rehabilitative period and short-term followup until 1 year because limited differences may be seen at longer term when no difference was observed initially. In addition, our study was limited to some degree by the intrinsic limitations common to all motion analysis studies, including variability in gait measurements resulting from body anthropometrics, independent skin motion, and definition of the neutral position [22]. Finally, we have chosen to focus on clinical results in terms of clinical outcomes and gait analysis, but blood loss, time of surgery, and cost-effectiveness analysis are mandatory to completely evaluate UKA PSI potential.
In 2013, Dao Trong et al. [7] investigated the tibial component alignment of 28 medial UKAs implanted using patient-specific cutting blocks. CT analysis of implant positioning showed a high accuracy of coronal tibial implant position (0.3° ± 1.7°), posterior slope (1.1° ± 2.6°), and external rotation (1.5° ± 3.3°). There was no control group in this study. Jaffry et al. [11] investigated implant positioning among PSI UKA, conventional instrumented UKA, and robotic-assisted UKA; their results showed that PSI UKA provided more accurate positioning than conventional instrumentation, no difference between robot and PSI UKA, and finally concluded that PSI UKA took half the time as robotic-assisted UKA to implant. Recently, Kerens et al. [14] compared radiographic positioning of implants in 30 conventional Oxford UKA (Biomet Inc, Warsaw, IN, USA) compared with 30 patient-specific guided Oxford UKA. They found no statistically differences between the two groups, except for the positioning of the femoral component in the frontal plane (better in the PSI group). Our results sustained those of this previous randomized controlled study because we did not find any differences in terms of lower limb mechanical axis or component alignment in frontal and sagittal planes. However, we have limited our radiological analysis to postoperative radiographic analysis and an analysis of the rotational positioning of UKA implant should be performed based on CT or MRI evaluation.
To our knowledge, PSI UKA clinical outcomes have never been compared with conventional instrumentation devices in a randomized controlled fashion and only one study reported the early clinical outcomes of 44 PSI UKA (41 patients) [6]. Those authors reported improved Oxford knee score and Forgotten Joint Score without any complication at a mean followup of 24 months. However, there was no control group and such improvements would be expected even without the use of PSI. More, the authors of this study had to modify PSI planning intraoperatively 19 times for 44 cases (43%), which may limit the interpretation of their functional results. In our study, improvement was observed in both groups at 3 months and 1 year without any difference between the two groups. We had to recut the tibial plateau in 10% of our PSI UKA cases; this number is lower than in the Bell et al. [6] series reporting 43% of perioperative modifications, but higher than the Kerens et al. series (no perioperative modification in 30 PSI UKA) [14]. The number of intraoperative changes in our experience was limited but not null, and this may be related first to the time spent by the surgeon on edition and validation of the preoperative planning and second to the fact that we would not accept intraoperatively an inappropriate cut.
Although this study is to our knowledge the first to describe gait analysis of PSI UKA compared with conventional UKA, our results are generally similar to those of other studies evaluating gait after UKA. Webster et al. [21] presented in their series of 12 UKAs (12 patients; mean age 69 ± 8 years) their spatiotemporal results (velocity 1.38 ± 0.2 m/s, cadence 118 ± 7.5 step/min, stride 1.70 ± 0.14 m) at a mean followup of 22 months were similar to those we found 1 year after UKA. In our series, we did not find any difference at 1 year between the two groups.
To conclude, our observations suggest that PSI does not provide better component positioning accuracy in frontal or sagittal planes. Additionally, PSI in UKA does not confer any substantial advantage in function after UKA. These arguments can therefore not be used to justify the extra cost related to this technique.
References
Abdel MP, Parratte S, Blanc G, Pomero V, Viehweger E, Argenson J-NA. No benefit of patient-specific instrumentation in TKA on functional and gait outcomes: a randomized clinical trial. Clin Orthop Relat Res. 2014;472:2468–2476.
Ahlbäck SO. [Classification of hip joint arthosis] [in Swedish]. Nord Med. 1971;85:157–158.
Argenson J-N, Flecher X, Parratte S. [Mini-invasive implantation of an uni-compartmental medial knee prosthesis] [in French]. Rev Chir Orthopédique Réparatrice Appar Mot. 2006;92:193–199.
Argenson J-N, Parratte S, Flecher X, Aubaniac J-M. Unicompartmental knee arthroplasty: technique through a mini-incision. Clin Orthop Relat Res. 2007;464:32–36.
Badawy M, Espehaug B, Indrekvam K, Havelin LI, Furnes O. Higher revision risk for unicompartmental knee arthroplasty in low-volume hospitals. Acta Orthop. 2014;85:342–347.
Bell SW, Stoddard J, Bennett C, London NJ. Accuracy and early outcomes in medial unicompartmental knee arthroplasty performed using patient specific instrumentation. Knee. 2014;21(Suppl 1):S33–S36.
Dao Trong ML, Diezi C, Goerres G, Helmy N. Improved positioning of the tibial component in unicompartmental knee arthroplasty with patient-specific cutting blocks. Knee Surg Sports Traumatol Arthrosc. 2014 Jan 17 [Epub ahead of print].
Emerson RH, Higgins LL. Unicompartmental knee arthroplasty with the oxford prosthesis in patients with medial compartment arthritis. J Bone Joint Surg Am. 2008;90:118–122.
Felts E, Parratte S, Pauly V, Aubaniac J-M, Argenson J-N. Function and quality of life following medial unicompartmental knee arthroplasty in patients 60 years of age or younger. Orthop Traumatol Surg Res. 2010;96:861–867.
Fitz W. Unicompartmental knee arthroplasty with use of novel patient-specific resurfacing implants and personalized jigs. J Bone Joint Surg Am. 2009;91(Suppl 1):69–76.
Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;423:161–165.
Jaffry Z, Masjedi M, Clarke S, Harris S, Karia M, Andrews B, Cobb J. Unicompartmental knee arthroplasties: robot vs patient specific instrumentation. Knee. 2014;21:428–434.
Kennedy WR, White RP. Unicompartmental arthroplasty of the knee. Postoperative alignment and its influence on overall results. Clin Orthop Relat Res. 1987;221:278–285.
Kerens B, Schotanus MGM, Boonen B, Kort NP. No radiographic difference between patient-specific guiding and conventional Oxford UKA surgery. Knee Surg Sports Traumatol Arthrosc. 2014 Jan 26 [Epub ahead of print].
Kim KT, Lee S, Kim TW, Lee JS, Boo KH. The influence of postoperative tibiofemoral alignment on the clinical results of unicompartmental knee arthroplasty. Knee Surg Relat Res. 2012;24:85–90.
Kuroyanagi Y, Nagura T, Kiriyama Y, Matsumoto H, Otani T, Toyama Y, Suda Y. A quantitative assessment of varus thrust in patients with medial knee osteoarthritis. Knee. 2012;19:130–134.
Noble PC, Scuderi GR, Brekke AC, Sikorskii A, Benjamin JB, Lonner JH, Chadha P, Daylamani DA, Scott WN, Bourne RB. Development of a new Knee Society scoring system. Clin Orthop Relat Res. 2012;470:20–32.
Robertsson O, Ranstam J, Sundberg M, W-Dahl A, Lidgren L. The Swedish Knee Arthroplasty Register: a review. Bone Joint Res. 2014;3:217–222.
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96.
Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233.
Webster KE, Wittwer JE, Feller JA. Quantitative gait analysis after medial unicompartmental knee arthroplasty for osteoarthritis. J Arthroplasty. 2003;18:751–759.
Wegrzyn J, Parratte S, Coleman-Wood K, Kaufman KR, Pagnano MW. The John Insall award: no benefit of minimally invasive TKA on gait and strength outcomes: a randomized controlled trial. Clin Orthop Relat Res. 2013;471:46–55.
Wiik AV, Manning V, Strachan RK, Amis AA, Cobb JP. Unicompartmental knee arthroplasty enables near normal gait at higher speeds, unlike total knee arthroplasty. J Arthroplasty. 2013;28:176–178.
Zambianchi F, Digennaro V, Giorgini A, Grandi G, Fiacchi F, Mugnai R, Catani F. Surgeon’s experience influences UKA survivorship: a comparative study between all-poly and metal back designs. Knee Surg Sports Traumatol Arthrosc. 2014 Mar 30 [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at St Marguerite Hospital, Marseille, France.
About this article
Cite this article
Ollivier, M., Parratte, S., Lunebourg, A. et al. The John Insall Award: No Functional Benefit After Unicompartmental Knee Arthroplasty Performed With Patient-specific Instrumentation: A Randomized Trial. Clin Orthop Relat Res 474, 60–68 (2016). https://doi.org/10.1007/s11999-015-4259-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-015-4259-0