Abstract
Background
Intraarticular injections, mainly using long-lasting corticosteroid suspensions, have long been used to treat knee osteoarthritis. Viscosupplementation is a relatively new approach with injection of a variety of agents. When comparing viscosupplementation with intraarticular injections of corticosteroids from baseline to the fourth week, steroids have been more effective for pain relief. By the fourth week they provide similar relief, but beyond that viscosupplementation appears to provide greater pain reduction. The delayed onset of symptomatic improvement combined with reports of reactive synovitis may discourage physicians and patients.
Questions/Purposes
We therefore addressed three questions: Does the addition of triamcinolone to viscosupplementation (1) improve first-week pain and function compared with viscosupplementation alone, (2) diminish adverse effects of viscosupplementation alone, and (3) alter 6-month pain and function of viscosupplementation alone?
Methods
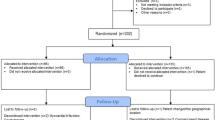
We prospectively enrolled 104 patients with knee osteoarthritis and randomized them to receive either a single intraarticular injection (6 mL) of hylan GF-20 (Group viscosupplementation [Group VS]), or a single intraarticular injection of hylan GF-20 (6 mL) and 1 mL (20 mg) of triamcinolone hexacetonide (Group VS + T). VAS, WOMAC™, and Lequesne questionnaires were completed at baseline and at Weeks 1, 4, 12, and 24.
Results
At Week 1 the WOMAC and VAS scores were lower in Group VS + T, compared with Group VS. There was no difference regarding the adverse effects. At Weeks 4, 12, and 24 there were no differences in the groups.
Conclusions
The addition of triamcinolone hexacetonide improves first-week symptom and functional scores of viscosupplementation, but not beyond. It does not seem to increase the likelihood of adverse effects.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.


Similar content being viewed by others
References
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049.
Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid: I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967;10:357–376.
Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis: meta-analysis. Osteoarthritis Cartilage. 2011;19:611–619.
Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704–1711.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;CD005328.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;CD005321.
Chevalier X, Jerosch J, Goupille P, van Dijk N, Luyten FP, Scott DL, Bailleul F, Pavelka K. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113–119.
Conaghan PG, Hunter DJ, Maillefert JF, Reichmann WM, Losina E. Summary and recommendations of the OARSI FDA osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis Cartilage. 2011;19:606–610.
DiBattista JA, Martel-Pelletier J, Wosu LO, Sandor T, Antakly T, Pelletier JP. Glucocorticoid receptor mediated inhibition of interleukin-1 stimulated neutral metalloprotease synthesis in normal human chondrocytes. J Clin Endocrinol Metab. 1991;72:316–326.
Divine JG, Zazulak BT, Hewett TE. Viscosupplementation for knee osteoarthritis: a systematic review. Clin Orthop Relat Res. 2007;455:113–122.
Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation: diagnosis and treatment. Clin Orthop Relat Res. 2004;419:130–137.
Gomis A, Miralles A, Schmidt RF, Belmonte C. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthritis Cartilage. 2009;17:798–804.
Guidolin DD, Ronchetti IP, Lini E, Guerra D, Frizziero L. Morphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500–730 kDa sodium hyaluronate (Hyalgan) and methylprednisolone acetate on primary osteoarthritis of the knee. Osteoarthritis Cartilage. 2001;9:371–381.
Hepper CT, Halvorson JJ, Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17:638–646.
Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints: comparative effects of and use of hydrocortisone as a local antiarthritic agent. JAMA. 1951;147:1629–1635.
Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, Reichmann WM, Losina E. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011;19:557–588.
Kellgren JH, Lawrence JS. Radiological assessment of rheumatoid arthritis. Ann Rheum Dis. 1957;16:485–493.
Kemper F, Gebhardt U, Meng T, Murray C. Tolerability and short-term effectiveness of hylan G-F 20 in 4253 patients with osteoarthritis of the knee in clinical practice. Curr Med Res Opin. 2005;21:1261–1269.
Lequesne MG. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol. 1997;24:779–781.
Lussier A, Cividino AA, McFarlane CA, Olszynski WP, Potashner WJ, De Medicis R. Viscosupplementation with hylan for the treatment of osteoarthritis: findings from clinical practice in Canada. J Rheumatol. 1996;23:1579–1585.
Navarro-Sarabia F, Coronel P, Collantes E, Navarro FJ, de la Serna AR, Naranjo A, Gimeno M, Herrero-Beaumont G. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70:1957–1962.
Ozturk C, Atamaz F, Hepguler S, Argin M, Arkun R. The safety and efficacy of intraarticular hyaluronan with/without corticosteroid in knee osteoarthritis: 1-year, single-blind, randomized study. Rheumatol Int. 2006;26:314–319.
Papachristou G, Anagnostou S, Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand Suppl. 1997;275:132–134.
Prieto JG, Pulido MM, Zapico J, Molina AJ, Gimeno M, Coronel P, Alvarez AI. Comparative study of hyaluronic derivatives: rheological behaviour, mechanical and chemical degradation. Int J Biol Macromol. 2005;35:63–69.
Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay JL, Bertrand C, Pelletier JP. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377.
Reichmann WM, Maillefert JF, Hunter DJ, Katz JN, Conaghan PG, Losina E. Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: a systematic review. Osteoarthritis Cartilage. 2011;19:550–556.
Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247.
Wang Y, Hall S, Hanna F, Wluka AE, Grant G, Marks P, Feletar M, Cicuttini FM. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195.
Yasuda T. Hyaluronan inhibits prostaglandin E2 production via CD44 in U937 human macrophages. Tohoku J Exp Med. 2010;220:229–235.
Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis: Part III. Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499.
Acknowledgments
We thank Rogerio Ruscitto do Prado MSc, for helping with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
The institution of all the authors has received, during the study period, funding from the São Paulo Research Foundation (FAPESP) (Sao Paulo, Brazil).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
de Campos, G.C., Rezende, M.U., Pailo, A.F. et al. Adding Triamcinolone Improves Viscosupplementation: A Randomized Clinical Trial. Clin Orthop Relat Res 471, 613–620 (2013). https://doi.org/10.1007/s11999-012-2659-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-012-2659-y