Abstract
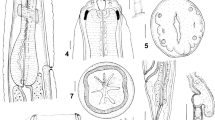
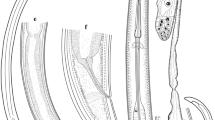
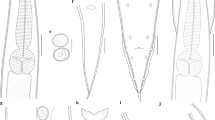
DNA sequencing of the nuclear ribosomal DNA internal transcribed spacers (ITS) and mitochondrial rrnS and cox2 genes, and analysis of polymorphisms in restriction profiles in the ITS and rrnS, were used to characterise anisakid nematodes belonging to Contracaecum Railliet & Henry, 1912 infecting the brown pelican Pelecanus occidentalis (L.) in Galveston Bay, Texas and Sarasota Bay, Florida. Molecular data led to the detection of two new species: Contracaecum fagerholmi n. sp., which was also supported by clear morphological evidence, and Contracaecum rudolphii F, a new cryptic species within the Contracaecum rudolphii Hartwich, 1964 complex. Bayesian phylogenetic analysis demonstrated that C. fagerholmi and C. rudolphii F form two well-separated clusters, with C. fagerholmi being closely related to Contracaecum bioccai Mattiucci et al., 2008 and C. rudolphii F being included in the C. rudolphii complex. C. fagerholmi can be readily differentiated morphologically from all of its congeners, other than C. microcephalum (Rudolphii 1809) and the five currently recognised members of the C. rudolphii complex (C. rudolphii A, B, C, D and E). C. fagerholmi differs from C. microcephalum in the length of the spicules and the shape of the distal tip of the spicules, and from C. rudolphii (sensu lato) in the shape and size of the ventro-lateral and dorsal lips and by having interlabia which are not distally bifurcate. Further studies are needed to determine which morphological characteristics can be used to distinguish the cryptic species of the C. rudolphii complex in order to assign them with formal names. The recovery of a third species, C. bioccai, from the brown pelican confirms its occurrence in this host and extends its known geographical distribution.






Similar content being viewed by others
References
Abollo, E., Paggi, L., Pascual, S., & D’Amelio, S. (2003). Occurrence of recombinant genotypes of Anisakis simplex s.s and Anisakis pegreffii (Nematoda: Anisakidae) in an area of sympatry. Infection, Genetics and Evolution, 3, 175–181.
Anderson, R. C. (2000). Nematode parasite of vertebrates, their development and transmission. (2nd Edit.) Wallingford: CAB International, 650 pp.
Barus, V., Sergeeva, T. P., Sonin, M. D., & Ryzhikov, K. M. (1978). Helminths of fish-eating birds of the Palaearctic Region. I. Nematoda. Prague: Academia, 318 pp.
Bunkley-Williams, L., & Williams, E. H. Jr. (1994). Parasites of Puerto Rican freshwater sport fishes. San Juan, Puerto Rico: Puerto Rico Department of Natural and Environmental Resources; and Mayaguez, Puerto Rico: Department of Marine Sciences, University of Puerto Rico, 164 pp.
Cavallero, S., Nadler, S. A., Paggi, L., Barros, N. B., & D’Amelio, S. (2011). Molecular characterization and phylogeny of anisakid nematodes from cetaceans from southeastern Atlantic coasts of USA, Gulf of Mexico, and Caribbean Sea. Parasitology Research, 108, 781–792.
Courtney, C. H., & Forrester, D. J. (1974). Helminth parasites of the brown pelican in Florida and Louisiana. Journal of the Helminthological Society of Washington, 41, 89–93.
Courtney, C. H., Forrester, D. J., & White, F. H. (1977). Anthelminthic treatment of brown pelicans. Journal of the American Veterinary Medicine Association, 171, 921–922.
D’Amelio, S., Mathiopoulos, K. D., Santos, C. P., Pugachev, O. N., Webb, S. C., Picanço, M., & Paggi, L. (2000). Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase chain reaction-based restriction fragment length polymorphism. International Journal for Parasitology, 30, 223–226.
D’Amelio, S., Barros, N. B., Ingrosso, S., Fauquier, D. A., Russo, R., & Paggi, L. (2007). Genetic characterization of members of the genus Contracaecum (Nematoda: Anisakidae) from fish-eating birds from west central Florida, U.S.A., with evidence of new species. Parasitology, 134, 1041–1051.
Deardorff, T. L., & Overstreet, R. M. (1980). Contracaecum multipapillatum (= C. robustum) from fishes and birds in the northern Gulf of Mexico. Journal of Parasitology, 66, 853–856.
Diaz-Ungria, C. (1978). Helminthos parasitos de vertebrados en el Estado Zulia. Algunas especies nuevas para Venezuela. Kasmera, 6, 207–233.
Diaz-Ungria, C. (1979). Algunas especies de helminthos nuevas para Venezuela. Revista Iberica de Parasitologia, 39, 313–336.
Dronen, N. O., Blend, C. K., & Anderson, C. K. (2003). Endohelminths from the brown pelican, Pelecanus occidentalis, and the American white pelican, Pelecanus erythrorhynchus, from Galveston Bay, Texas, U.S.A., and checklist of pelican parasites. Comparative Parasitology, 70, 140–154.
Drummond, A. J., & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7, 214.
Dyer, W. G., Williams, E. H., Jr, Mignucci-Giannoni, A. A., Jiménez-Marrero, N. M., Bunkley-Williams, L., Moore, D. P., & Pence, D. B. (2002). Helminth and arthropod parasites of the brown pelican, Pelecanus occidentalis, in Puerto Rico, with a compilation of all metazoan parasites reported from this host in the Western Hemisphere. Avian Pathology, 31, 441–448.
Fagerholm, H.-P. (1989). Intra-specific variability of the morphology in a single population of the seal parasite Contracaecum osculatum (Rudolphi) (Nematoda, Ascaridoidea), with a redescription of the species. Zoologica Scripta, 18, 33–41.
Fagerholm, H.-P. (1991). Systematic implications of male caudal morphology in ascaridoid nematode parasites. Systematic Parasitology, 19, 215–228.
Fagerholm, H.-P., & Overstreet, R. M. (2009). Ascaridoid nematodes: Contracaecum, Porrocaecum, and Baylisascaris. In: Atkinson, C. T., Thomas, N. J., & Hunter, D. B. (Eds) Parasitic diseases of wild birds. Oxford: Wiley-Blackwell, pp. 413–433.
Greve, J. H., Albers, H. F., Suto, B., & Grimes, J. (1986). Pathology of gastrointestinal helminthiasis in the brown pelican (Pelecanus occidentalis). Avian Diseases, 30, 482–487.
Grimes, J., Suto, B., Greve, J. H., & Albers, H. F. (1989). Effect of selected anthelminthics on three common helminths in the brown pelican (Pelecanus occidentalis). Journal of Wildlife Diseases, 25, 139–142.
Hartwich, G. (1964). Revision der vogel parasitischen Nematoden Mitteleuropas. II. Die Gattung Contracaecum Railliet & Henry, 1912 (Ascaridoidea). Mitteilungen aus dem Zoologischen Museum in Berlin, 40, 15–53.
Hu, M., D’Amelio, S., Zhu, X. Q., Paggi, L., & Gasser, R. B. (2001). Mutation scanning for sequence variation in three mitochondrial DNA regions for members of the Contracaecum osculatum (Nematoda: Ascaridoidea) complex. Electrophoresis, 22, 1069–1075.
Hugot, J. P., Morand, S., & Vassart, M. (1991). Morphological study of Contracaecum magnicollare (Nematoda, Anisakidae) from Anous minutus (Aves, Laridae). Systematic Parasitology, 20, 229–236.
Huizinga, H. W. (1966). Studies on the life cycle and development of Contracaecum spiculigerum (Rudolphi, 1809) (Ascaroidea: Heterocheilidae) from marine piscivorous birds. Journal of the Elisha Mitchell Scientific Society, 82, 181–195.
Huizinga, H. W. (1971). Contracaeciasis in pelecaniform birds. Journal of Wildlife Diseases, 7, 198–204.
Humphrey, S. R., Courtney, C. H., & Forrester, D. J. (1978). Community ecology of the helminth parasites of the brown pelican. The Wilson Bulletin, 90, 587–598.
Hutton, R. F. (1964). A second list of parasites from marine and coastal animals of Florida. Transactions of the American Microscopical Society, 83, 439–444.
Jacobs, D. E., Zhu, X. Q., Gasser, R. B., & Chilton, N. B. (1997). PCR-based methods for identification of potentially zoonotic ascaridoid parasites of the dog, fox and cat. Acta Tropica, 68, 191–200.
Li, A., D’Amelio, S., Paggi, L., He, F., Gasser, R. B., Lun, Z. R., Abollo, E., Turchetto, M., & Zhu, X. Q. (2005). Genetic evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) and the validity of Contracaecum septentrionale (Kreis, 1955) (Nematoda: Anisakidae). Parasitology Research, 96, 361–366.
Löytynoja, A., & Goldman, N. (2005). An algorithm for progressive multiple alignment of sequences with insertions. Proceedings of the National Academy of Sciences of the United States of America, 102, 10557–10562.
Mattiucci, S., Paoletti, M., Olivero-Verbel, J., Baldiris, R., Arroyo-Salgado, B., Garbin, L., Navone, G., & Nascetti, G. (2008). Contracaecum bioccai n. sp. from the brown pelican Pelecanus occidentalis (L.) in Colombia (Nematoda: Anisakidae): morphology, molecular evidence and its genetic relationship with congeners from fish-eating birds. Systematic Parasitology, 69, 101–121.
Mattiucci, S., Paoletti, M., Solorzano, A. C., & Nascetti, G. (2010). Contracaecum gibsoni n. sp. and C. overstreeti n. sp. (Nematoda: Anisakidae) from the Dalmatian pelican Pelecanus crispus (L.) in Greek waters: genetic and morphological evidence. Systematic Parasitology, 75, 207–224.
Mozgovoi, A. A. (1953). [Principles of nematodology II. Ascaridata of animals and man and the diseases caused by them. Part II.]. Moscow: Izdatel’stvo Akademii Nauk SSSR, 616 pp. (in Russian).
Nadler, S. A., D’Amelio, S., Fagerholm, H.-P., Berland, B., & Paggi, L. (2000). Phylogenetic relationships among species of Contracaecum Railliet & Henry, 1912 and Phocascaris Host, 1932 (Nematoda: Ascaridoidea) based on nuclear rDNA sequence data. Parasitology, 121, 455–463.
Nadler, S. A., & De Leon, G. P. (2011). Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology, 1, 1–22.
Nadler, S. A., & Hudspeth, D. S. S. (2000). Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. Journal of Parasitology, 86, 380–393.
Posada, D. (2009). Selection of models of DNA evolution with jModelTest. Methods in Molecular Biology, 537, 93–112.
Shamsi, S., Gasser, R., Beveridge, I., & Alizadeh Shabani, A. (2008). Contracaecum pyripapillatum n. sp. (Nematoda: Anisakidae) and a description of C. multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitology Research, 103, 1031–1039.
Shamsi, S., Norman, R., Gasser, R., & Beveridge, I. (2009). Genetic and morphological evidence for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitology Research, 105, 529–538.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Vincze, T., Posfai, J., & Roberts, R. J. (2003). NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Research, 31, 3688–3691.
Zhu, X. Q., Chilton, N. B., Jacobs, D. E., Boes, J., & Gasser, R. B. (1999). Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. International Journal for Parasitology, 29, 469–478.
Zhu, X. Q., D’Amelio, S., Gasser, R. B., Yang, T. B., Paggi, L., He, F., Lin, R. Q., Song, H. Q., Ai, L., & Li, A. X. (2007). Practical PCR tools for the delineation of Contracaecum rudolphii A and Contracaecum rudolphii B (Ascaridoidea: Anisakidae) using genetic markers in nuclear ribosomal DNA. Molecular and Cellular Probes, 21, 97–102.
Zhu, X. Q., D’Amelio, S., Hu, M., Paggi, L., & Gasser, R. B. (2001). Electrophoretic detection of population variation within Contracaecum ogmorhini (Nematoda: Ascaridoidea: Anisakidae). Electrophoresis, 22, 1930–1934.
Zhu, X. Q., D’Amelio, S., Paggi, L., & Gasser, R. B. (2000a). Assessing sequence variation in the internal transcribed spacers of ribosomal DNA within and among members of the Contracaecum osculatum complex (Nematoda: Ascaridoidea: Anisakidae). Parasitology Research, 86, 667–683.
Zhu, X. Q., D’Amelio, S., Palm, H. W., Paggi, L., George-Nascimento, M., & Gasser, R. B. (2002). SSCP-based identification of members within the Pseudoterranova decipiens complex (Nematoda: Ascaridoidea: Anisakidae) using genetic markers in the internal transcribed spacers of ribosomal DNA. Parasitology, 24, 615–623.
Zhu, X. Q., Gasser, R. B., Jacobs, D. E., Hung, G. C., & Chilton, N. B. (2000b). Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitology Research, 86, 738–744.
Zhu, X. Q., Gasser, R. B., Podolska, M., & Chilton, N. B. (1998a). Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. International Journal for Parasitology, 28, 1911–1921.
Zhu, X. Q., Jacobs, D. E., Chilton, N. B., Sani, R. A., Cheng, N. A., & Gasser, R. B. (1998b). Molecular characterization of a Toxocara variant from cats in Kuala Lumpur, Malaysia. Parasitology, 117, 155–164.
Acknowledgement
This paper is dedicated to the memory of one of the authors, a dear friend and colleague, Nélio B. Barros, whose essential contribution made this study possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
D’Amelio, S., Cavallero, S., Dronen, N.O. et al. Two new species of Contracaecum Railliet & Henry, 1912 (Nematoda: Anisakidae), C. fagerholmi n. sp. and C. rudolphii F from the brown pelican Pelecanus occidentalis in the northern Gulf of Mexico. Syst Parasitol 81, 1–16 (2012). https://doi.org/10.1007/s11230-011-9323-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-011-9323-x