Abstract
Fossil evidence of photosynthesis, documented in Precambrian sediments by microbially laminated stromatolites, cyanobacterial microscopic fossils, and carbon isotopic data consistent with the presence of Rubisco-mediated CO2-fixation, extends from the present to ~3,500 million years ago. Such data, however, do not resolve time of origin of O2-producing photoautotrophy from its anoxygenic, bacterial, evolutionary precursor. Though it is well established that Earth’s ecosystem has been based on autotrophy since its very early stages, the time of origin of oxygenic photosynthesis, more than 2,450 million years ago, has yet to be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Geological time is divided into two major segments: the Phanerozoic Eon, the younger and much shorter of the segments, which begins with the first appearance of shelly invertebrate animals ~542 million years (Ma) ago and includes the familiar evolutionary successions from algae to spore plants to naked-seed and then flowering plants, and from invertebrates to fish and then the rise of life on land; and the Precambrian Eon, the longer of the segments, which spans the earlier seven–eighths of Earth history, extending from the formation of the planet, ~4,500 Ma ago, to the beginning of the Phanerozoic. The Precambrian, in turn, is subdivided into two exceedingly long segments—each some 2,000 Ma in duration—the Archean, extending from the formation of the planet to 2,500 Ma ago, and the Proterozoic, spanning the time from 2,500 Ma ago to the beginning of the Phanerozoic. The oldest known fossils date from ~3,500 Ma ago (Schopf 1993, 2006; Schopf et al. 2007), with hints of life being present in ~3,830-Ma-old rocks, among the oldest known on Earth (Mojzsis et al. 1996; McKeegan et al. 2007).
Though it is likely that the earliest forms of life were heterotrophs, dependent on abiotically produced organics for their foodstuffs (Oparin 1938; summarized in Schopf 1999), evidence from the rock record (primarily, microbially produced stromatolites, cellular microscopic fossils, and the carbon isotopic composition of preserved organic matter) establishes that photoautotrophy—emerging first in photosynthetic prokaryotes—has served as the foundation of the world’s ecosystem since at least 3,500 Ma ago. The principal unsolved problem is not whether photosynthesis was an exceedingly ancient evolutionary innovation, but, rather, when did oxygen-producing photosynthesis originate, a metabolic process that arose as an evolutionary derivative of a more primitive form of photoautotrophy, anoxygenic photosynthesis, characteristic of non-cyanobacterial photosynthetic bacteria (Blankenship 1992; Blankenship and Hartman 1998). Among all major biological innovations, probably those of foremost evolutionary impact were the origin of eukaryotic sexuality (a hugely important development, ~1,000 Ma ago, which set the stage for the evolution of multicellular life; Schopf et al. 1973; Schopf 1999) and the much earlier development, originating in cyanobacteria, of O2-producing phototosynthesis, the advent of which altered the world’s ecosystem by providing the biologically available oxygen required for aerobic respiration, a decidedly more energetically efficient process than its anaerobic (fermentative) precursors (cf. Schopf 1999).
To establish the time of origin of oxygenic photosynthesis, a prime question needs to be answered, namely, “When did cyanobacteria first appear?” Firm fossil evidence of the existence of these microorganisms—the earliest-evolved “complete aerobes,” capable both of O2-producing photosynthesis and O2-consuming respiration—would establish that the sequence of metabolic innovations leading to their emergence (anaerobic heterotrophy, followed by anaerobic photoautotrophy and then aerobic autotrophy and heterotrophy) had by that time already evolved, giving rise to an ancient, but metabolically fully modern, ecosystem (Schopf 1996, 1999). Evidence to answer this question should be expected to be preserved in the Precambrian rock record. For example, as is shown here, stromatolites, microbially layered deposits dominated today by filamentous and coccoidal cyanobacteria, are present throughout virtually all of the known geological record; cellularly preserved fossils of cyanobacteria dominate the documented record of Precambrian life; and rock-derived carbon isotopic data are consistent with the presence of photosynthetic microorganisms back to ~3,500 Ma ago and, possibly, to >3,800 Ma ago. Nevertheless, as is also shown here, a firm answer to the question of the time of origin of oxygenic photosynthesis is not yet available: the earliest known stromatolites might have been formed by anoxygenic, rather than O2-producing, photosynthesizers; the cyanobacterium-like fossils in rocks ~3,500 Ma might be remnants of non-O2-producing microbes; and though a vast amount of carbon isotopic data are consistent with the presence of oxygenic photosynthesis as early as ~3,500 Ma ago, they do not rule out the possibility that the role of primary producer in the world’s most ancient ecosystems was played by anaerobic, anoxygenic, photosynthetic bacteria.
It should not be surprising that the question of time of origin of O2-producing photosynthesis (i.e., of cyanobacteria) is yet unresolved. In contrast with paleontological studies of the Phanerozoic history of life, the basic outlines of which were already known in the mid-1800s when they served as the basis for Darwin’s great tome on the Origin of Species, active investigation of the earlier, Precambrian, fossil record did not commence until the mid-1960s, more than a century later (Barghoorn and Schopf 1965; Barghoorn and Tyler 1965; Cloud 1965; Schopf 1968). And although great progress has been made in the ensuing decades (see, for example, Schopf and Bottjer 2009)—showing that Precambrian microbes were abundant, ubiquitous, metabolically diverse, and biotically predominant—knowledge of the early fossil record remains far from complete. Moreover, due to the “geologic cycle,” the repeated sequence of mountain building, erosion, and deposition into sedimentary basins of the eroded mineral grains thus produced, the average “lifetime” of a geological unit is only some 200 Ma. For this reason, the rock record that has survived to the present rapidly decreases with increasing geological age, a petering-out that severely limits the ancient fossil record available for study. Thus, it is estimated that about half of the potentially fossil-bearing sedimentary rocks that have survived to the present are of Phanerozoic age, the most recent one-eighth of geological time, whereas the remaining 50% date from the Precambrian, the earlier seven-eighths of Earth history, and that Archean-age rocks—those older than 2,500 Ma in which evidence of the earliest oxygenic photosynthesizers is expected to occur—represent only about 5% of the surviving rock mass (Garrels and Mackenzie 1971). Although the known fossil record of cellularly preserved microbes extends deep into the Precambrian—throughout all of the Proterozoic and much of the Archean—in units older than ~2,000 Ma it becomes increasingly sparse and patchy, and the history of the various microbial lineages becomes increasingly difficult to decipher.
The great oxidation event
Despite the problems posed by the petering-out of the rock and fossil records over geological time, the record that has survived is sufficient to establish the presence of molecular oxygen and, by implication, of oxygen-producing photoautotrophs, at least as early as ~2,450 Ma ago. As summarized by Holland (2002) and Canfield (2005), beginning about 2,200 Ma ago and continuing to the present, sandstones known as red beds have been deposited on land surfaces by meandering rivers and windblown dust. The beds are colored red by the presence of the mineral hematite (Fe2O3), iron oxide that typically forms a thin veneer on individual quartz sand gains and the presence of which indicates that the atmosphere at the time was oxidizing. In contrast, in numerous terrains older than about 2,400 Ma, conglomeratic rocks occur that contain detrital grains of pyrite and uraninite deposited in shallow-water deltaic settings, minerals that in the presence of molecular oxygen are rapidly converted to their oxidized forms—for pyrite (FeS2), to the mineral hematite (Fe2O3); and for uraninite (UO2), to its soluble more oxidized form, UO4. If there had been appreciable oxygen in the overlying atmosphere when these sediments were laid down, hematite, rather than pyrite, would occur in such conglomerates and uraninite would have oxidized and been dissolved.
The temporal distributions of red beds and of pyritic uraniferous conglomerates thus indicate that there was an increase in the amount of oxygen in Earth’s atmosphere some 2,200–2,400 Ma ago, a date that has recently been more firmly set by studies of sulfur isotopic ratios preserved in the rock record that evidence a major rise in atmospheric O2-content at ~2,450 Ma ago (Farquhar et al. 2000, 2007). Since photosynthesis produces well over 99% of the oxygen in the atmosphere, and since no other large-scale source of free oxygen is known, this increase of atmospheric O2 can be firmly attributed to the activities of oxygenic photosynthesizers. Nevertheless, the timing of this major increase, dubbed the Great Oxidation Event (Holland 2002), sets only a minimum age of ~2,450 Ma for the origin of O2-producing photosynthesis. As Earth’s primordial environment was anoxic, the molecular oxygen generated by the earliest oxygenic photosynthesizes would have been rapidly consumed, removed from the atmosphere by its reaction with previously unoxidized substrates (e.g., volcanic gases, unoxided minerals, and huge amounts of ferrous iron dissolved in the world’s oceans) to be buried in rock-forming minerals. Only after all such substrates had been completely oxidized could the content of atmospheric oxygen have permanently increased, a time lag from the origin of O2-producing photosynthesis that lasted several and perhaps many hundreds of millions of years.
Taken as a whole, the evidence available indicates that O2-producing photosynthetic microorganisms originated earlier than 2,450 Ma ago; that such microbes were likely in place by 2,700 Ma ago; and that the origin of oxygenic photosynthesis may date from as early as, or even earlier than, 3,500 Ma ago.
Paleobiological evidence of photosynthesis
Three principal lines of evidence are available to address the question of the time of origin of oxygenic photosynthesis—stromatolites, cellular microfossils, and the chemistry of ancient organic matter—each of which is discussed, in turn, below.
Stromatolites
As preserved in the geological record, stromatolites are finely layered rock structures, typically composed of carbonate minerals (e.g., calcite, CaCO3), that are formed by the microbially mediated accretion of laminae, layer upon layer, from the surface of an ancient seafloor or lake bottom. Their layered structure reflects the photosynthetic metabolism of the mat-building microorganisms. Thin (mm-thick) mats composed of such microbes formed as the microorganisms multiplied and spread across surfaces that were intermittently veneered by detrital or precipitated mineral grains that blocked sunlight. To maintain photosynthesis, mobile members of such communities, such as gliding oscillatoriacean cyanobacteria, moved upward through the accumulated mineral matter to establish a new, overlying, microbial mat. The repeated accretion and subsequent lithification of such mats, commonly augmented by an influx of non-mobile microbes (such as colonial chroococcacean, entophysalidacean, and pleurocapsacean cyanobacteria), can result in the formation of stromatolitic structures that range from small millimetric columns and pustular mounds to large, decimetric bioherms. During diagenesis, the series of changes that lead to the lithification and preservation of such structures, silica (quartz, SiO2), can replace the initially precipitated carbonate matrix. If replacement occurs early in the history of a deposit, before the mat-building microorganisms decay and disintegrate, cellularly intact microbes can be preserved. However, the vast majority of stromatolites, unaltered by such replacement, are devoid of fossil microbes: during diagenesis, carbonate grain growth crushes and obliterates the stromatolite-forming microorganisms, leaving only an amorphous thin coaly residuum of microbe-derived carbonaceous matter.
Carbonate microbial stromatolites occur today (Fig. 1a, b, d) that in size, shape, and laminar structure are much like those known from the Precambrian (Fig. 1c, e, f). Such modern stromatolites are usually restricted to refugia, environments such as hypersaline lagoons (Fig. 1a, b, d) in which the slow-growing microbial mats are not disrupted by grazing and burrowing metazoans. For this reason, stromatolites are not particularly abundant in sediments of the Phanerozoic, deposits laid down in environments dominated by diverse types of metazoans. But in the absence of grazing and burrowing animals, as was the situation until the very end of the Precambrian, stromatolites were abundant and morphologically varied in shallow-water carbonate deposits worldwide. Known earliest from rocks ~3,500 Ma in age, their distribution over time parallels that of the surviving Precambrian rock record—that is, stromatolite-bearing rock units become less and less abundant as the record of increasingly older rocks gradually peters out. Such structures establish the presence of flourishing photosynthesis-based microbial communities, but only rarely do they preserve the cellular fossils that might indicate whether the stromatolite-building photoautotrophs were oxygenic, like cyanobacteria, or anoxygenic, like photosynthetic bacteria.
Modern and fossil stromatolites. a Modern stromatolites at Shark Bay (Hamelin Pool), Western Australia. b Modern Shark Bay columnar and domical stromatolites for comparison with (c) fossil stromatolites from the ~2,300-Ma-old Transvaal Dolomite, Cape Province, South Africa. d–f Modern and fossil vertically sliced columnar to domical stromatolites showing upwardly accreted microbial laminae from Shark Bay (d), the ~1,300-Ma-old Belt Supergroup of Montana (e), and the ~3,350-Ma-old Fig Tree Group of the eastern Transvaal, South Africa (f). Scale for a and c shown by the geological hammers enclosed by red circles
Archean stromatolites
As is shown in Fig. 2, an impressive number of Archean-age geological units—of particular interest because of their potential bearing on the time of origin of oxygenic photosynthesis—are known to contain microbially produced stromatolites. Shown in Fig. 3 are representative examples: carbonate sediments of the ~2,723-Ma-old Fortescue Group of Western Australia contain domical, pseudocolumnar and branching stromatolites (Fig. 3a and b); those of the ~2,985-Ma-old Insuzi Group of South Africa include stratiform and conical forms (Fig. 3c and d); and those of the ~3,388-Ma-old Strelley Pool Chert of Western Australia contain close-packed conical stromatolites patchily distributed over many tens of square kilometers (Fig. 3e through g). The presence of conical stromatolites in such deposits, like those shown in Fig. 3c through g and reported from 17 of the 48 units listed in Fig. 2 (Hofmann et al. 1999; Hofmann 2000; Allwood et al. 2006, 2009, 2010; Schopf 2006), is particularly noteworthy since such distinctive structures evidently require for their formation “highly motile mat builders” such as oscillatoriacean cyanobacteria (Grotzinger and Knoll 1999, pp. 342–343).
Archean-age microbially laminated stromatolites. a Domical, pseudocolumnar and branching stromatolites, overlain by rippled sediments, and b a domical stromatolite from the ~2,723-Ma-old Tumbiana Formation (Fortescue Group) of Western Australia. c Conical stromatolite and d stratiform and conical stromatolites, from the ~2,985-Ma-old Insuzi Group, South Africa. e–g Laterally linked conical stromatolites from the ~3,388-Ma-old Strelley Pool Chert of Western Australia
Cellular fossils
Two principal processes preserve cellular microbial fossils: compression and permineralization. Compression-preserved microorganisms occur in fine-grained detrital sediments such as shales and siltstones, pressed and flattened along bedding planes as the sediment lithified. Such compression-preserved microbes are poorly known from the Phanerozoic, largely neglected by Phanerozoic paleontologists who focus chiefly on megascopic remains, but they are appreciably better documented in the Precambrian (e.g., Butterfield 2009).
The microbial fossil record is best known from microorganisms preserved by permineralization. Of all modes of fossil preservation, this process (known also as petrification) provides the most faithful representation of life-like morphology. Such preservation, common for plants and fungi as well as fossilized prokaryotes, results from the pervasion of mineral-charged solutions into cells during the early stages of diagenesis, prior to their decay and disintegration. The permeating fluids infill microscopic voids—replacing the watery milieu of the cellular components—to produce a mineral-infused inorganic–organic mix that preserves physically robust structures such as organic-rich cell walls. As a result, both the organismal morphology and cellular anatomy of such fossils can be preserved in microscopic detail. The most common such permineralizing matrix is silica, fine-grained (cryptocrystalline) quartz, the mineral that comprises the rock-type known as chert. Hundreds of microbe-preserving cherts are now known from the Precambrian when silica was abundant in the world’s oceans, well before the Phanerozoic appearance of silica-biomineralized sponges, diatoms and radiolarians that today regulate the oceanic silica budget. As shown here, such cherts can contain exquisitely preserved fossil microbes.
Filamentous cyanobacteria
Among the several taxonomic families of filamentous cyanobacteria, stromatolite-building members of the Oscillatoriaceae have the most extensive fossil record, represented by diverse fossils in hundreds of ancient microbial communities (e.g., Fig. 4a through q). Two representative Precambrian examples, ~850 Ma in age, are shown in Fig. 4a through f: a spirally coiled specimen (Helioconema funiculum, Fig. 4a and b), similar to species of the modern oscillatoriacean genus Spirulina; and a tapering cellular trichome (Cephalophytarion laticellulosum, Fig. 4c through f) that resembles the modern cyanobacterium Oscillatoria amoenum. The organismal form and cellular structure of such specimens, traditionally illustrated by photomicrographic montages (e.g., Fig. 4a and c), can be appreciably better documented by use of confocal laser scanning microscopy (CLSM), a technique only recently introduced to Precambrian studies (Schopf et al. 2006). Compare, for example, the optical image of the spirally coiled specimen (Fig. 4a) with its CLSM image (Fig. 4b), and the optical image of the tapering trichome, artificially flattened in the photomontage (Fig. 4c), with the corresponding CLSM images (Fig. 4d and e) that show the specimen to plunge steeply into the thin rock slice (a petrographic thin section) in which it is embedded. A second newly introduced technique, Raman imagery (Schopf et al. 2005), can be used to document, in three dimensions (Schopf and Kudryavtsev 2005), the chemical composition of such rock-embedded fossils and that of their embedding matrix, for the tapering trichome, showing that the walls of its terminal cells are composed of carbonaceous kerogen and that the cells themselves are permineralized by quartz (Fig. 4f).
Fossil oscillatoriacean cyanobacteria (a through f) in petrographic thin sections of stromatolitic chert from the ~850-Ma-old Bitter Springs Formation of central Australia; modern oscillatoriaceans (g and h) compared with a morphologically similar fossil trichome (i through q) in a thin section of a cherty stromatolite from the ~775 Ma-old Chichkan Formation of southern Kazakhstan; and pustular laminae, formed by colonies of entophysalidacean cyanobacteria, in a thin section of stromatolitic chert from the ~2,100-Ma-old Kasegalik Formation of the Belcher Islands, Canada. a, b Optical montage (a), composed of five photomicrographs (denoted by the white lines), and a CLSM image (b) of Heliconema, a spirally coiled oscillatoriacean similar to modern Spirulina. c–f Optical montage (c), composed of ten photomicrographs (denoted by the white lines), and CLSM (d and e) and a 3-D Raman image (f) of a large-celled specimen of Cephalophytarion that descends from where it transects the upper surface of the thin section (at the far right) to a depth of 20 μm (at the far left); the larger red rectangle in c denotes the area shown in e, whereas the smaller rectangle denotes the area shown in f; unlike the composite optical image (c), which shows only the medial plane of the specimen, the CLSM image (d) shows its true 3-D morphology; the 3-D Raman image of its end cells (f), rotated to show the flat uppermost surface of the cells where they transect the thin section surface, demonstrates that the kerogenous cell walls (gray) enclose quartz-filled cell lumina (white). g, h Optical images of two specimens of modern Oscillatoria sp. showing the rounded terminal cells (left), disk-shaped medial cells, and partial septations (arrows) characteristic of oscillatoriacean cyanobacteria. i Optical image of the fossil oscillatoriacean, Oscillatoriopsis media, descending into a thin section at a low angle from left to right, shown in a photomontage in which the red rectangles denote the areas of the trichome shown in CLSM images (j through n) and 3-D Raman images (o through q). j The trichome terminus, showing its rounded end-cell and subtending disk-shaped medial cells. k A part of the trichome situated ~14 μm deeper in the section than the trichome terminus (and ~28 μm below the upper surface of the section) that exhibits partial septations (arrows) like those shown in g and h. l–n A deeper part of the trichome (~39 μm below the upper surface of the section) that similarly exhibits partial septations (arrows), in l and m showing the specimen as viewed from above its upper surface (the same perspective as shown in i, but in m with the trichome tilted slightly to the right to show its interior) and in n showing the trichome as viewed from its side. o–q 3-D Raman images (acquired in a spectral window centered in the kerogen “G” band at ~1605 cm−1) showing the kerogenous composition of the trichome and its partial septations: o, the part of specimen denoted by the red rectangle in l, as viewed from above the trichome; p, the part denoted in m, titled slightly to the left; q, the part denoted in n, showing the specimen from its side. r A low-magnification optical image of stromatolitic laminae formed by laterally interlinked colonies (at arrows) of the entophysalidacean cyanobacterium Eoentophysalis
The trichomes of the great majority of members of the Oscillatoriaceae are characterized by rounded terminal cells, disk-shaped medial cells, and partial septations, incipient cell walls that grow inward to produce daughter cells (Fig. 4g and h). Although in fossil specimens such structures are not always evident by optical microscopy, CLSM and Raman imagery can establish their presence. For example, compare the photomicrographs of modern Oscillatoria sp. (Fig. 4g and h) with that of its fossil equivalent, Oscillatoriopsis media, shown in Fig. 4i in a thin section of chert from the ~775-Ma-old Chichkan Formation of southern Kazakhstan. Owing to the CLSM laser-induced fluorescence of the coaly kerogen (primarily, interlinked polycyclic aromatic hydrocarbons), which comprises the cell walls of the fossil, its detailed morphology is appreciably better defined in the CLSM images (Fig. 4j though n) than in the corresponding optical image (Fig. 4i), whereas 3-D Raman imagery documents the carbonaceous composition of its permineralized cells (Fig. 4o–q).
The cells of modern oscillatoriaceans divide by the centripetal invagination of partial septations that fuse in the center of a cell to produce transverse cell walls. The lateral cell walls of such trichomes are about twice the thickness of their transverse walls, and they contain rigidifying peptidoglycans that are absent from partial septations and transverse walls except at the cell periphery (Pankratz and Bowen 1963; Frank et al. 1971; Halfen and Castenholz 1971; Drews 1973). Owing to these differences, lateral cell walls tend to be relatively well preserved in fossil specimens whereas the thinner transverse walls, like their precursor partial septations, are typically preserved only in part. Despite these differences, use of CLSM to analyze fossil specimens shows the presence of such partial sepatations (Fig. 4k though n), with 3-D Raman imagery (Fig. 4o–q) confirming their carbonaceous composition. Not only do such data establish the oscillatoriacean affinities of these cellular trichomes, showing that they are morphologically essentially identical to living members of the family, but they indicate also that their cell division occurred by the same genetically determined processes as their modern counterparts. Data such as these show that the fossil record of the Oscillatoriaceae extends deep into geological time and that such cyanobacteria have changed little or not at all over thousands of millions of years (Schopf 1994a, 1999, 2009).
Coccoidal cyanobacteria
Although almost always of lesser abundance than filamentous microorganisms in Precambrian communities, coccoidal cyanobacteria, such as the entophysalidacean colonies shown in Fig. 4r from cherts of the ~2,100-Ma-old Kasegalik Formation of Canada, can be important mat-forming components. Entophysalidaceans (Fig. 5a and b), however, are generally less common than chroococcacean cyanobacteria (Fig. 5c and d), a great number of genera and species of which have been described from Precambrian deposits (Mendelson and Schopf 1992). Similarly, pleurocapsaceans, such as those shown in Fig. 4e and f, are common in many chert-permineralized Precambrian stromatolitic units.
Modern and fossil entophysalidacean, chroococcacean, and pleurocapsacean coccoidal and ellipsoidal cyanobacteria; all fossils are shown in petrographic thin sections of stromatolitic chert. a Modern Entophysalis sp. (Entophysalidaceae) for comparison with b Eoentophysalis belcherenisis from the ~2,100-Ma-old Kasegalik Formation of the Belcher Islands, Canada. c Modern Gloeocapsa sp. (Chroococcaceae) for comparison with d Gloeodiniopsis uralicus from the ~1,500-Ma-old Satka Formation of Baskiria, Russia. e Modern Pleurocapsa sp. (PCC 7327, Pleurocapsaceae) for comparison with f Paleopleurocapsa reniforma from the ~775-Ma-old Chichkan Formation of southern Kazakhstan
Archean microbes
As shown above, the fossil record of cyanobacteria—and, thus, of oxygenic photosynthesis—is well documented to ≥2,100 Ma ago. Though O2-producing photosynthesis originated appreciably earlier, exactly how much earlier remains to be established. Is this uncertainty due to the petering-out of the rock record (and the fossil-destroying metamorphic alteration to which the older surviving rocks have been subjected), or, rather, does the fossil record, as now known, evidence the true evolutionary history of this process? The Archean fossil record holds the answer.
Fossils classed as Bacteria Incertae Sedis—that is, fossil prokaryotes of the Bacterial Domain that cannot be referred with certainty to a particular bacterial group—are known throughout the geological record. Such remnants constitute the great majority of the fossils now known from Archean-age rocks. Owing to the geological recycling discussed above, only about 5% of rocks exposed at the Earth’s surface date from the Archean (Garrels and Mackenzie 1971) and, accordingly, the record of Archean fossils is sparse, in the interval between 2,500 and 3,500 Ma reported from only some 40 rock units and comprising only six broad bacterium-like morphotypes (Schopf 2006). Of these geological units, 14 date from the interval between 3,200 and 3,500 million years ago, evidence that well documents the existence of microbe-level life this early in Earth history. For virtually all such ancient microbes, the uncertainty in their classification stems from their morphological similarity both to cyanobacteria and non-cyanobacterial bacteria. Given such uncertainty, however, they cannot resolve the question of the time of O2-producing photosynthesis.
The Archean fossil microbes most studied are those of the ~3,465-Ma-old Apex chert of northwestern, Western Australia (Schopf 1992a, 1993, 1999; Schopf et al. 2002, 2007, 2010). Shown in Fig. 6 are specimens of Primavifilum amoenum, one of 11 taxa of microorganisms described from this unit (Schopf 1993). These microscopic fossils, and many, but not all, of the ten other taxa reported from the deposit, are “cyanobacterium-like” in their morphology and cellular anatomy (e.g., compare Fig. 6a through c with Fig. 4a and c). Nevertheless, because of microbial mimicry—the occurrence of more or less identical morphologies in taxa of oxygenic and non-oxygen-producing microbes (Schopf 1992b, 1999)—organismal and cellular morphology, in and of themselves, cannot provide firm evidence of the physiological capabilities of such very ancient microbes (Schopf 1993). What is needed to resolve such uncertainty is an Archean fossil record like that of the Proterozoic, one sufficiently continuous and well documented that it unambiguously links younger fossils of well-established affinities to their older, and typically less well-preserved, evolutionary precursors.
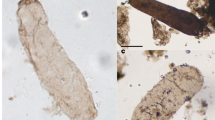
Thin section-embedded filamentous microbes from the ~3,465-Ma-old Apex chert of northwestern Western Australia. a–d Optical images of three specimens of Primaevifilum amoenum, in c and d showing two views of the same specimen situated 3–9 μm below the thin section surface; the red rectangle in c denotes the part shown in e through j; the arrows in d, an optical image of the surface of the thin section with the specimen outlined in black, point to the variously shaped quartz grains of the embedding chert matrix, the irregularity of which shows that the specimen is not a pseudofossil produced by the organic coating of mineral grains. e 3-D Raman image; the organic (carbonaceous, kerogenous) filament (gray) is cylindrical and, like younger Precambrian cellular fossils (e.g., Fig. 3 q), is composed of quartz-filled cells (white). f–j 2-D Raman images at sequential depths below the filament surface (f, at 0.75 μm; g, 1.5 μm; h, 2.25 μm; i, 3.0 μm; j, 3.75 μm); arrows in f point to quartz-filled cell lumina (black) defined by kerogenous cell walls (white), evident also in g through j
Given the forgoing summaries of the fossil records of Precambrian stromatolites and microfossils, it is easily conceivable that Earth’s biota 3,500 Ma ago was based on oxygen-producing photoautotrophy. Nevertheless, neither of these lines of evidence can rule out the possibility that the primary producers in Earth’s earliest ecosystems were anaerobic, non-O2-producing, photoautotrophs. In an effort to resolve this question, we will now turn to the data provided by the chemistry of preserved Precambrian organic matter.
Carbonaceous matter
Hydrocarbon biomarkers
Extraction, isolation, and identification by gas chromatography–mass spectroscopy of organic biomarkers, particularly of various types of hydrocarbons, have provided useful insight into the nature of Proterozoic life. For example, identification of the protozoan biomarker tetrahymenol in ~930-Ma-old sediments (Summons 1992), supported by the presence of fossil testate amoebae in the same sedimentary sequence (Bloeser et al. 1977; Bloeser 1985; Schopf 1992c; Porter and Knoll 2000), has established a minimum age for the Proterozoic emergence of protozoan protists.
Few such studies have been carried out on older, Archean-age rocks, of which the most notable is the report of steranes (hydrogenated derivatives of steroids, such as cholesterol) identified in extracts of ~2,700-Ma-old carbonaceous shales of northwestern Australia (Brocks et al. 1999). This finding is unexpected, since steroids occur almost exclusively in eukaryotic cells (Summons et al. 2006), principally as components of cellular membranes, and assured fossil eukaryotes (large-celled spheroidal phytoplankton) are known earliest from sediments ~1,800 Ma in age (Schopf 1992c) which are nearly a billion years younger than the sterane-containing rocks. However, if the reported steranes date from ~2,700 Ma ago, their occurrence would seem to indicate that molecular oxygen must have been present in the local environment—since steroid biosynthesis involves numerous O2-requiring enzyme-mediated steps (for cholesterol, 11 such steps, beginning with the cyclization of squalene; Schopf 1978; Summons et al. 2006). This, in turn, would imply that O2-producing photosynthesizers must also have been present, since there appears to be no other plausible source for production of the free oxygen required.
The interpretation of these biomarkers is complicated. Although it seems clear that the sterane-containing shales have been dated correctly, potential contamination from modern sources (e.g., from drilling fluids or introduced during laboratory analyses) is an ever-present problem in such studies. Moreover, all organic compounds are soluble to some extent in ground water and for this reason can be introduced into rocks long after their deposition, from not only modern but also geologically ancient sources. As there are no techniques by which to determine directly the age of organic compounds extracted from ancient sediments, it is difficult to show definitively that such organics are syngenetic with the rock in which they occur.
Owing to these and related problems, Rasmussen et al. (2008) suggested that the Australian shale-associated steranes are much younger than ~2,700 Ma, most probably less than ~2,200 Ma in age. However, subsequent, more detailed studies that correlate the distribution of these biomarkers with their carbon isotopic compositions and their differing paleoecological settings provide convincing evidence that they are syngenetic with rocks from which they have been reported (Eigenbrode et al. 2008). And these results showing the syngenicity of such biomarkers with their enclosing sediments have now been duplicated in studies of essentially the same suite of biomarkers extracted from multiple horizons of South African rock units ~2,600 Ma in age obtained from two boreholes geographically separated by some 24 km (Waldbauer et al. 2009).
Taken together, the available data indicate that sterane biomarkers date to ~2,700 Ma ago, well before the Great Oxidation Event of the early Proterozoic. As such, these biomarkers represent strong presumptive evidence of O2-producing photoautotrophy.
Kerogen: particulate carbonaceous organic matter
In contrast to extractable biomarkers, kerogen, the insoluble particulate organic matter of ancient sediments—occurring either as the carbonaceous constituent of cellularly preserved fossils, such as those discussed above, or as finely divided dispersed particles—is immobile, locked within its embedding rock matrix. In all carbonaceous rocks, whether Phanerozoic or Precambrian and whether or not they contain identifiable fossils, the kerogen occurs entirely or almost entirely as bits and pieces of carbonaceous detritus. As such kerogen is demonstrably syngenetic with its encompassing mineral matrix, and because it comprises the great bulk of the carbonaceous matter in sedimentary rocks, most analyses of Precambrian organic matter, and virtually all studies of Archean organic matter, have focused on the chemistry of kerogen. Three types of analyses, discussed below, have proven useful: (1) Raman spectroscopy of its molecular structure; (2) solid-state 13C nuclear magnetic resonance and X-ray absorption near-edge spectroscopy studies of its elemental composition and functional groups; and (3) mass spectrometric measurements of its carbon isotopic composition.
Raman spectroscopy of individual fossils
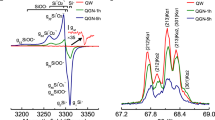
As illustrated above (Fig. 4f and o through q; Fig. 6e through j), 2- and 3-D Raman imagery provide firm evidence of the carbonaceous composition of cellularly preserved Precambrian microorganisms. In addition, however, the Raman spectra on which such images are based can themselves be analyzed to determine quantitatively the geochemical maturity of the preserved organic matter. Shown in Fig. 7 are Raman spectra acquired from the kerogenous cell walls of representative fossil microbes permineralized in eight Precambrian geological units ~720 to ~3,465 Ma in age. The spectra shown—ordered from less (top) to more (bottom) geochemically mature and representative of a much larger suite of kerogen-comprised microfossils for which such data are available (Schopf et al. 2005)—were acquired from microfossils preserved in rocks that range from relatively little metamorphosed (top) to being appreciably more geologically altered (bottom), metamorphosed to middle greenschist facies. As the spectra illustrate, the two principal Raman bands of kerogen change markedly as its molecular structure, altered primarily by heat, progresses along a geochemical pathway toward graphite: as the carbonaceous matter becomes structurally more ordered, the left-most (“D”) band becomes increasingly narrow and more peaked and the right-most (“G”) band narrows and, in partially graphitized kerogen, ultimately bifurcates.
Raman spectra of the kerogenous cell walls of representative Precambrian microfossils permineralized in cherts of the ~850-Ma-old Bitter Springs, ~1900-Ma-old Gunflint, ~775 Ma-old Chichkan, and ~1050-Ma-old Allamoore Formations, the ~3,465-Ma-old Apex chert, the ~760-Ma-old Skillogalee and ~720-Ma-old Auburn Dolomites, and the ~775-Ma-old River Wakefield Formation (Schopf et al. 2005, 2007), ordered by their RIP values (Schopf et al. 2005) from less (top) to more (bottom) geochemically mature
For each of the eight spectra shown in Fig. 7 is listed its Raman Index of Preservation (RIP) value, a quantitative measure of the organic geochemical maturity of the analyzed kerogen that reflects the local geological (diagenetic and metamorphic) environment to which the fossil-containing unit has been subjected (Schopf et al. 2005). Of rapidly increasing use in paleobiological studies (e.g., Chen et al. 2007; Schopf et al. 2008; Schopf and Kudryavtsev 2009; Igisu et al. 2009) and derived directly from the Raman spectra measured, such RIP values are highly reproducible and easily calculated (Schopf et al. 2005). Such data, providing a quantitative measure of the quality of organic preservation unavailable by any other technique, are useful for assessing the syngenicity of organic-walled fossils and their associated mineral matrices and can provide convincing evidence of the biological origin of the carbonaceous matter studied (Schopf et al. 2005, 2008). In and of themselves, however, they do not indicate the metabolic characteristics (e.g., whether autotrophic or heterotrophic) of the individual fossils analyzed.
NMR- and XANES-analyses of particulate kerogen
Analyses by 13C nuclear magnetic resonance (NMR) of pyrolysates of kerogen isolated from the ~3,490-Ma-old Towers Formation of northwestern Western Australia document the presence of aliphatic carbon moieties (CH2 and CH3), aromatic C=C (present in the polyaromatic hydrocarbons of which such kerogens are predominately composed; Schopf et al. 2005), and both C–O and C=O groups (Derenne et al. 2008). The Derenne et al. (2008) study also records the presence in such pyrolysates of an homologous series of long chain (C10–C18) aliphatic hydrocarbons that are characterized by an odd-over-even carbon number predominance, “a unique characteristic of organics formed biologically since it reflects biosynthesis using addition of C2 units” (Derenne et al. 2008, p. 479). The biological origin of kerogen preserved in the ~3,565-Ma-old Apex chert, also of northwestern Western Australia and the source of the cellular filamentous Archean microbes illustrated in Fig. 6, is similarly well documented. Using X-ray absorption near-edge spectroscopy (XANES), backed by numerous other techniques, DeGregorio et al. (2009) carried out a comparative study of the Apex kerogen and that of the famous and assuredly microfossil-bearing (Barghoorn and Tyler 1965; Cloud 1965) ~1,900-Ma-old Gunflint chert of southern Ontario, Canada. The results show that—rather being abiotic organic matter produced by Fischer–Tropsch-type syntheses, as postulated by Brasier et al. (2002)—the Apex kerogen contains all of the biogenic elements (carbon, hydrogen, oxygen, nitrogen, sulfur and phosphorous: CHONSP) as well as functional groups, such as “carboxyl [–COOH] and phenol [Caromatic–OH] peaks” (DeGregorio et al. 2009, p. 632), that are typical of biologically derived kerogen. Based on their exceptionally detailed study, DeGregorio et al. (2009, p. 632) conclude that “Apex carbonaceous matter and Gunflint kerogen are chemically complex… [both containing] similar amounts of nitrogen, sulfur, and phosphorous [in which the presence of phosphorus, in particular] implies a biogenic origin.”
The Derenne et al. (2008) and DeGregorio et al. (2009) studies establish, convincingly, the biological origin of the kerogen analyzed: as expressed by Derenne et al. (2008, p. 480), the “data report the occurrence of biological markers in the kerogen embedded in a 3.5 By old chert, [an] observation that supports a scenario according to which life was present on Earth 3.5 By ago”; and DeGregorio et al. (2009, p. 631) conclude that available data imply “that the Apex microbe-like features represent authentic biogenic organic matter”. Despite the confirmation of the biogenicity of the particulate kerogen and of the kerogenous Apex microfossils that these studies report, they do not provide evidence of the metabolic characteristics of the early Archean biota.
The carbon isotopic signature of photosynthesis
Spurred by the pioneering studies of Park and Epstein (1963) and Hoering (1967), data have been amassed from thousands of analyses of the carbon isotopic compositions of inorganic carbonate minerals and carbonaceous kerogens coexisting in Precambrian sediments (e.g., Strauss and Moore 1992). Such data show a consistent difference between the inorganic and organic carbon analyzed in the relative abundances of the two stable isotopes of carbon, 12C and 13C, which extends from the present to ~3,500 Ma ago (Fig. 8). The enrichment of the fossil organic matter in the lighter isotope, 12C, relative to coexisting carbonate (a proxy for the seawater-dissolved CO2 required for its precipitation) and the magnitude of the isotopic difference (expressed as δ13CPDB values) between the inorganic and organic carbon reservoirs, invariably falling within a range of 25 ± 10‰, are consistent with the carbon isotopic fractionation that occurs as a result of Rubisco-(ribulose bisphospate carboxylase/oxygenase-) mediated CO2-fixation in O2-producing cyanobacteria (e.g., Hayes et al. 1992; House et al. 2000, 2003). Such evidence of carbon isotopic fractionation is well documented in rocks ~3,200 to ~3,500 Ma in age, the oldest fossil-bearing deposits now known (Fig. 9).
Carbon isotopic values of coexisting carbonate and organic carbon measured in bulk samples of Phanerozoic and Precambrian sedimentary rocks, for the Precambrian represented by data from 100 fossiliferous cherts and shales shown as average values for groups of samples from 50-Ma-long intervals (Strauss and Moore 1992; Schopf 1994b)
Carbon isotopic values of carbonate and organic carbon measured in bulk samples of the oldest microfossiliferous units now known (Schopf 2006)
Although this carbon isotopic signature of photosynthesis seems certain to evidence the continuous existence of photoautotrophs over the past 3,500 Ma, it does not necessarily reflect the presence of oxygenic photoautotrophy. Owing to the mixing of carbonaceous matter from diverse biological sources which occurs as sediments are deposited, and the alteration of carbon isotopic compositions that can occur during geological metamorphism, the δ13CPDB values of the analyzed kerogen range broadly (±10‰) and, thus, are consistent not only with primary production by cyanobacteria but by non-O2-producing photosynthetic bacteria and, perhaps, anaerobic chemosynthetic bacteria. Archean kerogens may have been derived from some or all of these sources, and interpretation of the data is further complicated by the presence in Archean sediments of carbonaceous matter so enriched in 12C as to be plausibly derived only from CH4-metabolizing methanotrophs, indicating that methane-producing Archaea played a significant role in the ancient ecosystem (Hayes 1983; Schopf 1994b).
Carbon isotopic measurements of individual fossils
The carbon isotopic data summarized above were obtained on carbonate minerals and bulk samples of carbonaceous residues isolated by acid-maceration from large (kg-sized) rock specimens. Such analyses do not permit correlation of the isotopic values measured with the kerogen comprising individual microscopic fossils, the cellular morphology of which might be expected to provide evidence of their affinities and, thus, their metabolic capabilities. This deficiency has been addressed by use of secondary ion mass spectrometry (SIMS), a technique permitting direct measurement of the isotopic composition of the kerogenous cell walls of individual fossils, which has been applied to Precambrian microorganisms ranging from ~850 to nearly 3,500 Ma in age (Fig. 10 ). A technique that has been used both for the isotopic analyses (House et al. 2000; Ueno et al. 2001a, b) and elemental mapping (Oehler et al. 2009) of such fossils, the consistency between the δ13CPDB values measured by SIMS on individual microfossils and those obtained by conventional mass spectrometry on bulk kerogens from the same rock samples demonstrates the efficacy of the technique (Fig. 10). Recently, McKeegan et al. (2007) have used SIMS to establish the presence of 12C-rich graphitic carbon in the oldest sedimentary rocks now known, from Akilia Island off southwestern Greenland, the carbon isotopic composition of which (δ13CPDB-29 ± 4‰) suggests that autotrophic microbes may have existed as early as ~3,830 Ma ago.
Carbon isotopic values of individual Precambrian microfossils measured by secondary ion microprobe spectrometry (SIMS) compared with those of the carbonate and total organic carbon measured in bulk samples of the same geological units. Values plotted for carbonate and total organic carbon are from Strauss and Moore (1992); for microfossils from the Bitter Springs and Gunflint Formations, from House et al. (2000); and those for microfossils from the Dresser Formation, from Ueno et al. (2001a)
Despite such progress and the now-established paleobiological usefulness of SIMS, evidence provided by this technique does not resolve the question of the time of origin of oxygen-producing photosynthesis. As yet, the SIMS-based data are too few and too imprecise to show definitively whether the individual fossils analyzed were oxygenic or anoxygenic photoautotrophs (cf. House et al. 2000), and the results even of the most recently published such isotopic work (McKeegan et al. 2007) can only hint at the presence of autotrophs ~3,830 Ma ago since it remains to be established whether the graphite analyzed dates from the time of deposition of the metasediment in which it occurs or was formed later, during the severe metamorphism to which the Akilia rocks have been subjected.
Summary and conclusions
As is shown in the foregoing discussion of the geological record of stromatolites, microscopic fossils, and the chemistry of the organic matter preserved in Precambrian rocks, the Earth’s ecosystem has been driven by photosynthesis from at least 3,500 Ma ago. It is equally clear, however, that the data now available do not indicate when O2-producing photosynthetic cyanobacteria evolved from their anoxygenic photosynthetic bacterial precursors. The presence throughout much of Earth history of microbially laminated stromatolites, cyanobacterial and cyanobacterium-like microfossils, and of carbon isotopic compositions of carbonate and kerogenous carbon that fit both the direction and magnitude of the isotopic fractionation produced by modern oxygenic photoautotrophy are consistent with, but are insufficient to establish the time of origin of O2-producing photosynthesis. Thus, the earliest, Archean, stromatolites might have been formed by phototaxic anoxygenic photosynthetic bacteria, rather than by the cyanobacteria that dominate the upper surfaces of such structures today. Similarly, and despite the prevalence of assured cyanobacterial microscopic fossils in relatively young, Proterozoic, Precambrian sediments, the filamentous and coccoidal microfossils of Archean terrains might represent remains of non-O2-producing photosynthesizers. And though the chemistry of ancient, Archean, organic matter shows it to be unquestionably biogenic, the carbon isotopic data available from such sediments, backed even by voluminous data from younger deposits, cannot discriminate between its possible oxygenic and anoxygenic photosynthetic sources.
It is certain that O2-producing photosynthesis evolved earlier, and perhaps much earlier, than the rise of atmospheric oxygen in the Great Oxidation Event of ~2,450 Ma ago (Farquhar et al. 2000, 2007; Holland 2002; Canfield 2005), but how much earlier has yet to be established.
Abbreviations
- CLSM:
-
Confocal laser scanning microscopy
- NMR:
-
13C nuclear magnetic resonance.
- RIP:
-
Raman index of preservation
- Rubisco:
-
Ribulose bisphosphate carboxylase-oxygenase
- SIMS:
-
Secondary ion mass spectrometry
- XANES:
-
X-ray absorption near-edge spectroscopy
References
Allwood AC, Walter MR, Kamber BS, Marshall CP, Burch IW (2006) Stromatolite reef from the Early Archaean era of Australia. Nature 441:714–718
Allwood AC, Grotzinger JP, Knoll AH, Burch IW, Anderson MS, Coleman ML, Kanik I (2009) Controls on development and diversity of Early Archean stromatolites. Proc Natl Acad Sci USA 106:9548–9555
Allwood AC, Kamber BS, Walter MR, Burch IW, Kanik I (2010) Trace elements record depositional history of an Early Archean stromatolitic carbonate platform. Chem Geol 270:148–163
Barghoorn ES, Schopf JW (1965) Microorganisms from the late Precambrian of central Australia. Science 150:337–339
Barghoorn ES, Tyler SA (1965) Microorganisms from the Gunflint chert. Science 147:563–577
Blankenship RE (1992) Origin and early evolution of photosynthesis. Photosynth Res 33:91–111
Blankenship RE, Hartman H (1998) The origin and evolution of oxygenic photosynthesis. Trends Biochem Sci 23(3):94–97
Bloeser B (1985) Melanoclyrillium, a new genus of structurally complex late Proterozoic microfossils from the Kwagunt Formation (Chuar Group), Grand Canyon, Arizona. J Paleontol 59:741–765
Bloeser B, Schopf JW, Horodyski RJ, Breed WJ (1977) Chitinozoans from the Late Precambrian Chuar Group of the Grand Canyon, Arizona. Science 195:676–679
Brasier MD, Green OR, Jephcoat AP, Kleppe AK, Van Kranendonk MJ, Lindsay JF, Steele A, Grassineau NV (2002) Questioning the evidence of Earth’s oldest fossils. Nature 416:76–81
Brocks JJ, Logan GA, Buick R, Summons RE (1999) Archean molecular fossils and the early rise of eukaryotes. Science 285:1033–1036
Butterfield NJ (2009) Modes of pre-Ediacaran multicellularity. Precam Res 173:201–211
Canfield DE (2005) The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu Rev Earth Planet Sci 33:1–36
Chen J-Y, Schopf JW, Bottjer DJ, Zhang C-Y, Kudryavtsev AB, Tripathi AB, Wang X-Q, Yang Y-H, Gao X, Yang Y (2007) Raman spectra of a ctenophore embryo from southwestern Shaanxi, China. Proc Natl Acad Sci USA 104:6289–6292
Cloud P (1965) Significance of the Gunflint (Precambrian) microflora. Science 148:27–45
DeGregorio BT, Sharp TG, Flynn GJ, Wirick S, Hervig RL (2009) Biogenic origin for Earth’s oldest putative fossils. Geology 37:631–634
Derenne S, Robert F, Skrzypczak-Bonduelle A, Gourier D, Binet L, Rouzaud J-N (2008) Molecular evidence for life in the 3.5 billion year old Warrawoona chert. Earth Planet Sci Lett 272:476–480
Drews G (1973) Fine structure and chemical composition of the cell envelopes. In: Carr NG, Whitton BA (eds) The biology of blue-green algae. University of California Press, Berkeley, pp 99–116
Eigenbrode JL, Freeman KH, Summons RE (2008) Methylhopane biomarker hydrocarbons in Hamersley Province sediments provide evidence for Neoarchean aerobiosis. Earth Planet Sci Lett 273:323–331
Farquhar J, Bao H, Thiemens M (2000) Atmospheric influence of Earth’s earliest sulfur cycle. Science 289:756–759
Farquhar J, Peterson M, Johnson DT, Strauss H, Masterson A, Weichert U, Kaufman AJ (2007) Isotopic evidence for Mesoarchaean anoxia and changing atmospheric sulfur chemistry. Nature 449:706–709
Frank H, Lefort M, Martin HH (1971) Elektronenoptische und chemische Untersuchungen an Zellwäden der Baaualgen, Phormidium unicinatum. Zeit Natur B 17:262–268
Garrels RM, Mackenzie FT (1971) Evolution of sedimentary rocks. Norton, New York
Grotzinger JP, Knoll AH (1999) Stromatolites in Precambrian carbonates: evolutionary mileposts or environmental dipsticks? Annu Rev Earth Planet Sci 27:313–358
Halfen LN, Castenholz RW (1971) Gliding motility in the blue-green alga, Oscillatoria princeps. J Phycol 7:133–145
Hayes JM (1983) Geochemical evidence bearing on the origin of aerobiosis, a speculative hypothesis. In: Schopf JW (ed) Earth’s earliest biosphere. Princeton University Press, Princeton, pp 291–301
Hayes JM, DesMarais DJ, Lambert IA, Strauss H, Summons RE (1992) Proterozoic biogeochemistry. In: Schopf JW, Kelin C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 81–134
Hoering TC (1967) The organic geochemistry of Precambrian rocks. In: Abelson PH (ed) Researches in geochemistry, vol 2. Wiley, New York, pp 87–111
Hofmann HJ (2000) Archean stromatolites as microbial archives. In: Riding RE, Awramik SM (eds) Microbial sediments. Springer, Berlin, pp 315–327
Hofmann HJ, Grey K, Hickman AH, Thorpe RI (1999) Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geol Soc Am Bull 111:1256–1262
Holland HD (2002) Volcanic gases, black smokers, and the great oxidation event. Geochim Cosmochim Acta 66:3811–3826
House CH, Schopf JW, McKeegan KD, Coath CD, Harrison TM, Stetter KO (2000) Carbon isotopic composition of individual Precambrian microfossils. Geology 28:707–710
House CH, Schopf JW, Stetter KO (2003) Carbon isotopic signatures of biochemistry: fractionation by archaeans and other thermophilic prokaryotes. Organ Geochem 34:345–356
Igisu M, Ueno Y, Shimojima M, Nakashima S, Awramik SM, Ohta H, Maruyama S (2009) Micro-FTIR spectroscopic signatures of Bacterial lipids in Proterozoic microfossils. Precam Res 173:19–26
McKeegan KD, Kudryavtsev AB, Schopf JW (2007) Raman and ion microscopic imagery of graphitic inclusions in apatite from the >3830 Ma Akilia supracrustals, West Greenland. Geology 35:383–397
Mendelson CV, Schopf JW (1992) Proterozoic and selected Early Cambrian microfossils and microfossil-like objects. In: Schopf JW, Klein C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 865–951
Mojzsis S, Arrenhius G, McKeegan KD, Nutman AP, Friend CRL (1996) Evidence for life on Earth before 3,800 million years ago. Nature 384:55–59
Oehler DZ, Robert F, Walter MR, Sugitani K, Allwood A, Meibom A, Mostefaoui S, Selo M, Thomen A, Gibson EK (2009) NanoSIMS: insights to biogenicity and syngeneity of Archaean carbonaceous structures. Precam Res 173:70–78
Oparin AI (1938) The origin of life. McMillian, New York
Pankratz HS, Bowen CC (1963) Cytology of blue-green algae. I. The cells of Symploca muscorum. Am J Bot 50:387–399
Park R, Epstein S (1963) Carbon isotopic fractionation during photosynthesis. Geochim Cosmochim Acta 21:110–115
Porter SM, Knoll AH (2000) Testate amoebae in the Neoproterozoic Era: evidence from vase-shaped microfossils in the Chuar Group, Grand Canyon. Paleobiol 26:360–385
Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR (2008) Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455:1101–1104
Schopf JW (1968) Microflora of the Bitter Springs formation, late Precambrian, central Australia. J Paleontol 42:651–688
Schopf JW (1978) The evolution of the earliest cells. Scient Am 239:110–138
Schopf JW (1992a) Paleobiology of the Archean. In: Schopf JW, Klein C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 25–39
Schopf JW (1992b) Proterozoic prokaryotes: affinities, geologic distribution, and evolutionary trends. In: Schopf JW, Klein C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 195–218
Schopf JW (1992c) Evolution of the Proterozoic biosphere: benchmarks, tempo, and mode. In: Schopf JW, Klein C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 583–600
Schopf JW (1993) Microfossils of the Early Archean Apex chert: new evidence of the antiquity of life. Science 260:640–646
Schopf JW (1994a) Disparate rates, differing fates: the rules of evolution changed from the Precambrian to the Phanerozoic. Proc Natl Acad Sci USA 91:6735–6742
Schopf JW (1994b) The oldest known records of life: stromatolites, microfosssils, and organic matter from the Early Archean of South Africa and Western Australia. In: Bengtson S (ed) Early life on Earth. Columbia University Press, New York, pp 193–206
Schopf JW (1996) Metabolic memories of Earth’s earliest biosphere. In: Marshall CR, Schopf JW (eds) Evolution and the molecular revolution. Jones and Bartlett, Boston, pp 73–105
Schopf JW (1999) Cradle of life: the discovery of Earth’s earliest fossils. Princeton University Press, Princeton
Schopf JW (2006) Fossil evidence of Archaean life. Phil Trans R Soc B 361:869–885
Schopf JW (2009) Paleontology, microbial. In: Lederberg J, Schaechter M (eds) Encyclopedia of microbiology, 3rd edn. Elsevier, Amsterdam, pp 390–400
Schopf JW, Bottjer DJ (2009) World summit on ancient microscopic fossils. Precam Res 173:1–3
Schopf JW, Kudryavtsev AB (2005) Three-dimensional Raman imagery of Precambrian microscopic organisms. Geobiology 3:1–12
Schopf JW, Kudryavtsev AB (2009) Confocal laser scanning microscopy and Raman imagery of ancient microscopic fossils. Precam Res 173:39–49
Schopf JW, Haugh BN, Molnar RE, Satterthwait DF (1973) On the development of metazoans and metaphytes. J Paleontol 47:1–9
Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD (2002) Laser-Raman imagery of Earth’s earliest fossils. Nature 416:73–76
Schopf JW, Kudryavtsev AB, Agresti DG, Czaja AD, Wdowiak TJ (2005) Raman imagery: a new approach to assess the geochemical maturity and biogenicity of permineralized Precambrian fossils. Astrobiology 5:333–371
Schopf JW, Tripathi AB, Kudryavtsev AB (2006) Three-dimensional optical confocal imagery of Precambrian microscopic organisms. Astrobiology 1:1–16
Schopf JW, Kudryavtsev AB, Czaja AD, Tripathi AB (2007) Evidence of Archean life: stromatolites and microfossils. Precam Res 158:141–155
Schopf JW, Tewari VC, Kudryatsev AB (2008) Discovery of a new chert permineralized microbiota of the Proterozoic Buxa Formation of the Ranjit Window, Sikkim, N.E. India, and its astrobiological implications. Astrobiology 8:735–746
Schopf JW, Kudryavtsef AB, Sugitani K, Walter MR (2010) Precambrian microbe-like pseudofossils: a promising solution to the problem. Precam Res 179:191–205
Strauss H, Moore TB (1992) Abundances and isotopic compositions of carbon and sulfur species in whole rock and kerogen samples. In: Schopf JW, Klein C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 709–798
Summons RE (1992) Abundance and composition of extractable organic matter. In: Schopf JW, Klein C (eds) The Proterozoic biosphere. Cambridge University Press, New York, pp 101–115
Summons RE, Bradley AS, Janke LL, Waldbauer JR (2006) Steroids, triterpenoids and molecular oxygen. Phil Trans Roy Soc B 361:951–968
Ueno Y, Isozaki Y, Yurimoto H, Maruyama S (2001a) Carbon isotopic signatures of individual Archean microfossils (?) from Western Australia. Internatl Geol Rev 40:196–212
Ueno Y, Maruyama S, Isozaki Y, Yuimoto H (2001b) Early Archaean (ca. 3.5 Ga) microfossils and 13C-depleted carbonaceous matter in the North Pole area, Western Australia: field occurrence and geochemistry. In: Nakasima S, Maruyama S, Brack A, Windley BF (eds) Geochemistry and the origin of life. Universal Academic Press, New York, pp 203–236
Waldbauer JR, Sherman LS, Sumner DY, Summons RE (2009) Late Archean molecular fossils from the Transvaal Supergroup record the antiquity of microbial diversity and aerobiosis. Precambrian Res 169:28–47
Acknowledgments
I thank J. Shen-Miller, A.B. Kudryavtsev, and C. Shi for reviews of this manuscript. This study is supported by CSEOL, the IGPP Center for the Study of Evolution and the Origin of Life at UCLA.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
William Schopf, J. The paleobiological record of photosynthesis. Photosynth Res 107, 87–101 (2011). https://doi.org/10.1007/s11120-010-9577-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-010-9577-1