Abstract
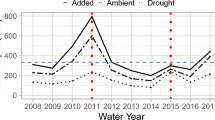
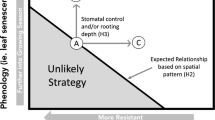
Patterns in soil moisture availability affect plant survival, growth and fecundity. Here we link patterns in soil moisture to physiological and demographic consequences in Florida scrub plants. We use data on different temporal scales to (1) determine critical soil moisture content that leads to loss of turgor in leaves during predawn measurements of leaf water status (Ψ crit), (2) describe the temporal patterns in the distribution of Ψ crit, (3) analyze the strength of relationship between rainfall and soil moisture content based on 8 years of data, (4) predict soil moisture content for 75 years of rainfall data, and (5) evaluate morphological, physiological and demographic consequences of spring 2006 drought on dominant shrubs in Florida scrub ecosystem in the light of water-uptake depth as determined by stable isotope analysis (δ18O). Based on 1998–2006 data, the soil moisture content at 50 cm depth explained significant variation in predawn leaf water potential of two dominant shrubs, Quercus chapmanii and Ceratiola ericoides (r 2 = 0.69). During 8 years of data collection, leaves attained Ψ crit only during the peak drought of 2000 when the soil moisture fell below 1% by volume at 50 and 90 cm depth. Precipitation explained a significant variation in soil moisture content (r 2 = 0.62). The patterns in predicted soil moisture for 75 year period, suggested that the frequency of drought occurrence has not increased in time. In spring 2006, the soil reached critical soil moisture levels, with consequences for plant growth and physiological responses. Overall, 24% of plants showed no drought-induced damage, 51% showed damage up to 50%, 21% had intense leaf shedding and 2% of all plants died. Over the drought and recovery period (May–October 2006), relative height growth was significantly lower in plants with greater die-back. All species showed a significant depression in stomatal conductance, while all but deep-rooted palms Sabal etonia and Serenoa repens showed significantly lower predawn (Ψ pd) and mid-day (Ψ md) leaf water potential in dry compared to wet season. Plants experiencing less severe die-back exhibited greater stomatal conductance, suggesting a strong relationship between physiology and morphology. Based on results we suggest that the restoration efforts in Florida scrub should consider the soil moisture requirements of key species.






Similar content being viewed by others
Abbreviations
- g s :
-
stomatal conductance
- Ψ pd :
-
predawn leaf water potential
- Ψ crit :
-
predawn leaf water potential at turgor loss
- Ψ md :
-
mid-day leaf water potential
- PWP:
-
permanent wilting point
- RWC:
-
relative water content, δ18O = (R sample / R SMOW − 1) × 1,000, in which R sample and R SMOW represent the heavy to light isotopes ratio of the sample and standard (Vienna standard mean ocean water) respectively
References
Abrahamson WG, Abrahamson CR (2002) Persistent palmettos: effects of 2000–2001 drought on Sabal etonia and Serenoa repens in Florida scrub ecosystem. Fla Sci 65:281–289
Abrahamson WG, Johnson AF, Layne JN, Peroni PA (1984) Vegetation of the Archbold Biological Station, Florida: an example of the southern Lake Wales Ridge. Fla Sci 47:209–250
Abrams MD, Menges ES (1992) Leaf ageing and plateau effects on seasonal pressure–volume relationships in three sclerophyllous Quercus species in south–eastern USA. Funct Ecol 6:353–360
Allen CD, Breshears DD (1998) Drought-induced shift of a forest–woodland ecotone: rapid landscape response to climate variation. Ecology 95:14839–14842
Ameglio T, Archer P, Cohen M, Valancogne C, Daudet FA, Dayau S et al (1998) Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant Soil 207:155–167
Asensio D, Penuelas J, Ogaya R, Llusia J (2007) Seasonal soil and leaf CO2 exchange rates in a Mediterranean Holm oak forest and their responses to drought conditions. Atmos Environ 41:2447–2455
Barton AM, Teeri JA (1993) The Ecology of elevational positions in plants: drought resistance in five montane pine species in southeastern Arizona. Am J Bot 80:5–25
Borchert R (1994) Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75:1437–1449
Boughton EA, Quintana-Ascencio P, Boughton RJ, Menges ES (2006) Association of ecotones with relative elevation and fire in an upland Florida landscape. J Veg Sci 17:361–368
Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG et al (2005) Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA 102:15144–15148
Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol 132:2166–2173
Brodribb TJ, Holbrook NM, Gutierrez MV (2002) Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ 25:1435–1444
Brown RB, Stone E, Carlisle V (1990) Soils. In: Myers RL, Ewel JJ (eds) Ecosystems of Florida. University of Central Florida Press, Orlando, pp 35–69
Buckley TN (2005) The control of stomata by water balance. New Phytol 168:275–291
Carter JL, Lewis D, Crockett L, Vega J (1989) Soils survey of Highlands County, Florida. National Cooperative Soil Survey, Orlando
Chen E, Gerber JF (1990) Climate. In: Myers RL, Ewel JJ (eds) Ecosystems of Florida. University of Central Florida Press, Orlando, pp 11–34
Ciais PM, Reichstein N, Viovy A, Granier J, Ogee V, Allard M et al (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
Comstock J, Mencuccini M (1998) Control of stomatal conductance by leaf water potential in Hymenoclea salsola (T. & G.), a desert subshrub. Plant Cell Environ 21:1029–1038
da Silva CR, Folegatti MV, da Silva TJA, Alves J, Souza CF, Ribeiro RV (2005) Water relations and photosynthesis as criteria for adequate irrigation management in ‘Tahiti’ lime trees. Sci Agricola 62:415–422
Damesin C, Rambal S (1995) Field study of leaf photosynthetic performance by a Mediterranean deciduous oak tree (Quercus pubescens) during a severe summer drought. New Phytol 13:159–167
Donovan LA, Linton MJ, Richards JH (2001) Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129:328–335
Ehleringer JR, Dawson TE (1992) Water uptake by plants—perspectives from stable isotope composition. Plant Cell Environ 15:1073–1082
Enfield DB, Mestas-Nunez AM, Trimble PJ (2001) The Atlantic multidecadal oscillation and its relation to rainfall and river flows in the continental US. Geophys Res Lett 28:2077–2080
Fay PA, Carlisle JD, Danner BT, Lett MS, McCarron JK, Stewart C et al (2002) Altered rainfall patterns, gas exchange, and growth in grasses and forbs. Int J Plant Sci 163:549–557
Fensham RJ, Fairfax RJ (2007) Drought related tree death of savanna eucalypts: species susceptibility, soil conditions and root architecture. J Veg Sci 18:71–80
Franks PJ, Drake PL, Froend RH (2007) Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ 30:19–30
Gebrekirstos A, Teketay D, Fetene M, Mitlohner R (2006) Adaptation of five co-occurring tree and shrub species to water stress and its implication in restoration of degraded lands. For Ecol Manage 229:259–267
Gitlin AR, Sthultz CM, Bowker MA, Stumpf SA, Paxton CA, Kennedy K et al (2006) Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought events. Conserv Biol 20:1477–1486
Gobron N, Pinty B, Melin F, Taberner M, Verstraete MM, Belward A et al (2005) The state of vegetation in Europe following the 2003 drought. Int J Remote Sens 26:2013–2020
Griffin JR (1973) Xylem sap tension in three woodland oaks of central California. Ecology 54:152–159
Hawkes CV, Casper BB (2002) Lateral root function and root overlap among mycorrhizal and nonmycorrhizal herbs in a Florida shrubland measured using rubidium as a nutrient analog. Am J Bot 89:1289–1294
Ho MD, Rosas JC, Brown KM, Lynch JP (2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32:737–748
Holbrook NM, Sinclair TR (1992) Water-balance in the arborescent palm, Sabal palmetto. 1. Stem structure, tissue water release properties and leaf epidermal conductance. Plant Cell Environ 15:393–399
Jones HG (1998) Stomatal control of photosynthesis and transpiration. J Exp Bot 49:387–398
Jones HG (2007) Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. J Exp Bot 58:119–130
Jones PD, Mann ME (2004) Climate over past millennia. Rev Geophys 42:RG2002
Kanemasu ET, Tanner CB (1969) Stomatal diffusion resistance of snap beans. I. Influence of leaf water potential. Plant Physiol 44:1547–1552
Kerr RA (2000) A North Atlantic climate pacemaker for the centuries. Science 288:1984–1986
Klujn N, Black TA, Griffis TJ, Barr AG, Gaumont-Guay D, Morgenstern K et al (2006) Response of net ecosystem productivity of three boreal forest stands to drought. Ecosystems (N Y, Print) 9:1128–1144
Koide RT, Robichaux RH, Morse SR, Smith CM (1989) Plant water status, hydraulic resistance and capacitance. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant Physiological Ecology-Field Methods and Instrumentation. Chapman and Hall, New York, pp 161–178
Krishnan P, Black TA, Grant NJ, Barr AG, Hogg ETH, Jassal RS et al (2006) Impact of changing soil moisture distribution on net ecosystem productivity of a boreal aspen forest during and following drought. Agric For Meteorol 139:208–223
Lambers H, Stuart C III, Pons TI (1998) Plant physiological ecology. Springer, New York
Leuzinger S, Zotz G, Asshoff R, Körner C (2005) Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol 25:641–650
Li JH, Johnson DP, Dijkstra P, Hungate BA, Hinkle CR, Drake BG (2007) Elevated CO2 mitigates the adverse effects of drought on daytime net ecosystem CO2 exchange and photosynthesis in a Florida scrub-oak ecosystem. Photosynthetica 45:51–58
Lite SJ, Stromberg JC (2005) Surface water and ground-water thresholds for maintaining Populus–Salix forests, San Pedro River, Arizona. Biol Conserv 125:153–167
Mann ME (2007) Climate over the past two millennia. Annu Rev Earth Planet Sci 35:111–136
Menges ES (1999) Ecology and conservation of Florida scrub. In: Anderson RC, Fralish JS, Baskin J (eds) Savannas, barrens, and rock outcrop plant communities of North America. Cambridge University Press, Cambridge, pp 7–22
Menges ES (2007) Integrating demography and fire management: an example from Florida scrub. Aust J Bot 55:261–272
Myers R (1990) Scrub and high pine scrub. In: Myers RL, Ewel JJ (eds) Ecosystems of Florida. University of Central Florida Press, Orlando, pp 150–193
Nepstad DC, Moutinho P, Dias MB, Davidson E, Cardinot G, Markewitz D, Figueiredo R, Vianna N, Chambers J, Ray D, Guerreiros JB, Lefebvre P, Sternberg L, Moreira M, Barros L, Ishida FY, Tohlver I, Belk E, Kalif K, Schwalbe K (2002) The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J Geophys Res Atmospheres 107(D20):8085
Nobel PS (1999) Physiochemical and environmental plant physiology, 2nd edn. Academic, San Diego
Orwig DA, Abrams MD (1997) Variation in radial growth responses to drought among species, site, and canopy strata. Trees (Berl) 11:474–484
Otieno DO, Schmidt MWT, Kinyamario JI, Tenhunen J (2005) Responses of Acacia tortilis and Acacia xanthophloea to seasonal changes in soil water availability in the savanna region of Kenya. J Arid Environ 62:377–400
Otieno DO, Kurz-Besson C, Liu J, Schmidt MWT, Do RVL, David TS et al (2006) Seasonal variations in soil and plant water status in a Quercus suber L. Stand: roots as determinants of tree productivity and survival in the Mediterranean-type ecosystem. Plant Soil 283:119–135
Padilla FM, Pugnaire FI (2007) Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct Ecol 21:489–495
Pellegrino A, Lebon E, Voltz M, Wery J (2004) Relationships between plant and soil water status in vine (Vitis vinifera L.). Plant Soil 266:129–142
Petrie CL, Hall AE (1992) Water relations in cowpea and pearl millet under soil water deficits. III. Extent of predawn equilibrium in leaf water potential. Aust J Plant Physiol 19:601–609
Pinheiro HA, DaMatta FM, Chaves ARM, Loureiro ME, Ducatti C (2005) Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Ann Bot (Lond) 96:101–108
Piper FI, Corcuera LJ, Alberdi M, Lusk C (2007) Differential photosynthetic and survival responses to soil drought in two evergreen Nothofagus species. For Sci 64:447–452
Rodriguez-Iturbe I, Porporato A, Laio F, Ridolfi L (2001) Intensive or extensive use of soil moisture: plant strategies to cope with stochastic water availability. Geophys Res Lett 28:4495–4497
Rundel PW, Jarrell WM (1989) Water in the environment. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology—field methods and instrumentation. Chapman and Hall, New York, pp 29–56
Saha S, Bassirirad H, Gladwin J (2005) Phenology and water relations of tree sprouts and seedlings in a tropical deciduous forest of South India. Trees (Berl) 19:322–325
Sakcali MS, Ozturk M (2004) Eco-physiological behaviour of some Mediterranean plants as suitable candidates for reclamation of degraded areas. J Arid Environ 57:141–153
Silva JS, Rego FC (2003) Root distribution of a Mediterranean shrubland in Portugal. Plant Soil 255:529–540
Sutton RT, Hudson LR (2005) Atlantic forcing of North American and European summer climate. Science 309:115–118
Swemmer AM, Knapp AK, Smith MD (2006) Growth responses of two dominant C4 grass species to altered water availability. Int J Plant Sci 167:1001–1010
Tardieu F, Davies WJ (1993) Integration of hydraulic and chemical signaling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ 16:341–349
Tyree MT, Jarvis PG (1982) Water in tissues and cells. In: Lange OS, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, new series vol. 12B. Springer, Berlin, pp 35–77
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, Berlin
Warwick RA (1975) Drought hazard in the United States: a research assessment. University of Colorado, Monograph No. NSF/RA/E-75/004
Weekley CW, Gagnon D, Quintana-Ascencio P, Menges ES, Saha S (2007) Variation in soil moisture in relation to rainfall, vegetation, gaps, and time-since-fire in Florida scrub. Ecoscience 14:71–80
Wunderlin RP, Hansen BF (2004) Atlas of Florida vascular plants. [S. M. Landry and K. N. Campbell (application development), Florida Center for Community Design and Research.] Institute for Systematic Botany, University of South Florida, Tampa. http://www.plantatlas.usf.edu/
Xu H, Li Y, Xu GQ, Zou T (2007) Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ 30:399–409
Zhang JH, Davies WJ (1989) Sequential response of whole plant water relations to prolonged soil drying and the involvement of xylem sap ABA in the regulation of stomatal behavior of sunflower plants. New Phytol 113:167–174
Acknowledgements
The authors thank E. Boughton, A. Catenazzi, F. Nicklen, A. Saha, J. Schafer and C. Weekley for comments on the manuscript. The study was funded by National Science Foundation’s grant (DEB98-15370) to ESM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tibor Kalapos.
Rights and permissions
About this article
Cite this article
Saha, S., Strazisar, T.M., Menges, E.S. et al. Linking the patterns in soil moisture to leaf water potential, stomatal conductance, growth, and mortality of dominant shrubs in the Florida scrub ecosystem. Plant Soil 313, 113–127 (2008). https://doi.org/10.1007/s11104-008-9684-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9684-3