ABSTRACT
Purpose
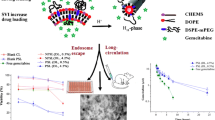
The objective of this study was to develop high-content gemcitabine PEGylated liposomes to reverse gemcitabine resistance in pancreatic tumour cells. The mechanism of drug loading into liposomes was also investigated.
Methods
To increase the drug entrapment efficiency (EE) and drug loading (DL), a novel passive loading approach named Small Volume Incubation method (SVI) was developed and compared to the reverse phase evaporation (REV) and remote loading methods. The in vitro cytotoxicity was evaluated using MIA PaCa-2 and Panc-1 cell lines.
Results
The EE for remote loading was 12.3 ± 0.3%, much lower than expected and a burst release was observed with the resultant liposomes. Using the optimized SVI method, increased EE (37 ± 1%) and DL (4%, w/w) were obtained. The liposomes (200 ± 5 nm) showed minimal drug leakage, good stability, and significant improvement in cytotoxicity to the gemcitabine-resistant pancreatic cancer cell lines.
Conclusions
Remote loading was not suitable for loading gemcitabine into liposomes. pKa > 4.6 for basic drugs and intra-liposomal precipitation of loaded compounds were suggested as an additional requirement to the current criteria for remote loading using ammonium sulphate gradient (pKa < 11). High DL is essential for liposomes to reverse gemcitabine resistance in pancreatic cell lines.




Similar content being viewed by others
Abbreviations
- DL:
-
Drug loading
- EE:
-
Entrapment efficiency
- MDR:
-
Multidrug resistance
- MTT:
-
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide
- PEG:
-
Polyethylene glycol
- REV:
-
Reverse phase evaporation
- SVI:
-
Small volume incubation
- TFH:
-
Thin film hydration
REFERENCES
Toscbi L, Finoccbiaro G, Bartolini S, Gioia V, Cappuzzo F. Role of gemcitabine in cancer therapy. Future Oncol. 2005;1:7–17.
Hilbig A, Oettle H. Gemcitabine in the treatment of metastatic pancreatic cancer. Expert Rev Anticancer Ther. 2008;8:511–23.
Mylonakis N, Athanasiou A, Ziras N, Angel J, Rapti A, Lampaki S, et al. Phase II study of liposomal cisplatin (LipoplatinTM) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer. 2010;68:240–7.
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–8.
Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol. 1997;24:S7-2–7.
Kohne AKC-H, Haufe TFJPT, Schleyer PGGEE. Pharmacokinetics of gemcitabine in a patient with end-stage renal disease: effective clearance of its main metabolite by standard hemodialysis treatment. Cancer Chemother Pharmacol. 2003;51:266–70.
Kuenen BBC, Rosen L, Smit EF, Parson MRN, Levi M, Ruijter R, et al. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol. 2002;20(6):1657–67.
Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70(9):3606–17.
Celia C, Grazia M, Paolino CD, Bulotta S, Ventura CA, Russo D, et al. Improved in vitro anti-tumoral activity, intracellular uptake and apoptotic induction of gemcitabine-loaded pegylated unilamellar liposomes. J Nanosci Nanotechnol. 2008;8:2102–13.
Kim I-Y, Kang Y-S, Lee DS, Park H-J, Choi E-K, Oh Y-K, et al. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J Control Release. 2009;140:55–60.
Federico C, Morittu VM, Britti D, Trapasso E, Cosco D. Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives. Int J Nanomedicine. 2012;7:5423–36.
Suzuki R, Takizawa T, Kuwata Y, Mutoha M, Ishiguro N, Utoguchi N, et al. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin–PEG-liposome. Int J Pharm. 2008;346:143–50.
Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci. 2001;6:66–77.
Slepushkin VA, Simões S, Dazin P, Newman MS, Guo LS, Pedroso de Lima MC, et al. Sterically stabilized pH-sensitive liposomes. J Biol Chem. 1997;272(4):2382–8.
Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995;1233:134–44.
K-i O, Una K, Minatoa K, Tanakab K-i, Higakia K, Kimura T. Determinants for in vivo anti-tumor effects of PEG liposomal doxorubicin: importance of vascular permeability within tumors. Int J Pharm. 2008;359:234–40.
Graeser R, Bornmann C, Esser N, Ziroli V, Jantscheff P, Unger C, et al. Antimetastatic effects of liposomal gemcitabine and empty liposomes in an orthotopic mouse model of pancreatic cancer. Pancreas. 2009;38:330–7.
Cosco D, Bulotta A, Ventura M, Celia C, Calimeri T, Perri G, et al. In vivo activity of gemcitabine-loaded PEGylated small unilamellar liposomes against pancreatic cancer. Cancer Chemother Pharmacol. 2009;64:1009–20.
Celano M, Calvagno MG, Bulotta S, Paolino D, Arturi F, Rotiroti D, et al. Cytotoxic effects of Gemcitabine-loaded liposomes in human anaplastic thyroid carcinoma cells. BMC Cancer. 2004;4(63):1–5.
Xua X, Khanb MA, Burgessa DJ. Predicting hydrophilic drug encapsulation inside unilamellar liposomes. Int J Pharm. 2012;432:410–8.
Bansal SS, Celia C, Ferrati S, Zabre E, Ferrari M, Palapattu G, et al. Validated RP-HPLC method for the simultaneous analysis of gemcitabine and LY-364947 in liposomal formulations. Curr Drug Targets. 2013;14:1–9.
Hong M-S, Lee SJ, Oh Y-K, Kim C-K. pH-sensitive, serum-stable and long-circulating liposomes as a new drug delivery system. J Pharm Pharmacol. 2002;54:51–8.
Zucker D, Marcus D, Barenholz Y, Goldblum A. Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties. J Control Release. 2009;139:73–80.
Bolotin EM, Cohen R, Bar LK, Emanuel N, Ninio S, Lasic DD, et al. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. J Liposome Res. 1994;4(1):455–79.
Cern A, Golbraikh A, Sedykh A, Tropsha A, Barenholz Y, Goldblum A. Quantitative structure -property relationship modeling of remote liposome loading of drugs. J Control Release. 2011. doi:10.1016/j.jconrel.2011.11.029.
Paolino D, Cosco D, Racanicchi L, Trapasso E, Celia C, Iannone M, et al. Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR®: biodistribution, pharmacokinetic features and in vivo antitumor activity. J Control Release. 2010;144:144–50.
Cosco D, Paolino D, Cilurzo F, Casale F, Fresta M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int J Pharm. 2012;422:229–37.
Calvagno MG, Celia C, Paolino D, Cosco D, Iannone M, Castelli F, et al. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr Drug Deliv. 2007;4:89–101.
Møkleby T. Active loading of gemcitabine into liposomes [Master]. University of Tromsø; 2009.
Gravem H. Gemcitabine-containing liposomes. University of Tromsø; 2006.
Vali AM, Toliyat T, Shafaghi B, Dadashzadeh S. Preparation, optimization, and characterization of topotecan loaded PEGylated liposomes using factorial design. Drug Dev Ind Pharm. 2008;34:10–23.
El-Gibaly I, Abdel-Ghaffar SK. Effect of hexacosanol on the characteristics of novel sustained-release allopurinol solid lipospheres (SLS): factorial design application and product evaluation. Int J Pharm. 2005;294:33–51.
Costa P. An alternative method to the evaluation of similarity factor in dissolution testing. Int J Pharm. 2001;220:77–83.
Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev. 2011;63:161–9.
Juliano RL, Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem Biophys Res Commun. 1975;63(3):651–8.
Zhanga T, Li Y, Mueller A. Phase structure of liposome in lipid mixtures. Chem Phys Lipids. 2011;164:722–6.
Garbuzenko O, Barenholz Y, Priev A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem Phys Lipids. 2005;135:117–29.
Cui J, Li C, Guo W, Li Y, Wang C, Zhang L, et al. Direct comparison of two pegylated liposomal doxorubicin formulations: is AUC predictive for toxicity and efficacy? J Control Release. 2007;118:204–15.
Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745:273–86.
Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol Membr Biol. 2010;27:190–205.
ACKNOWLEDGMENTS AND DISCLOSURES
The financial support for this study was provided by New Zealand Cancer Society (Grant Number 3627223). The authors also wish to acknowledge the support of a Doctorial Scholarship for Hongtao Xu provided by The University of Auckland, New Zealand. The authors declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Paxton, J., Lim, J. et al. Development of High-Content Gemcitabine PEGylated Liposomes and Their Cytotoxicity on Drug-Resistant Pancreatic Tumour Cells. Pharm Res 31, 2583–2592 (2014). https://doi.org/10.1007/s11095-014-1353-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1353-z