Abstract
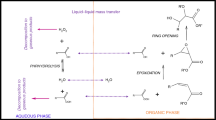
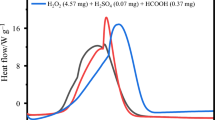
This article describes thermal risk assessment of vegetable oil epoxidation by peroxycarboxylic acid. It is a liquid–liquid system where several exothermic reactions occur. Acetic acid was used as the carboxylic acid, and oleic acid was chosen as a model molecule because it is a common fatty acid in the triglyceride molecule. Differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) were used to determine safety criteria such as the final temperature (T Final), T D24, and time to maximum rate under adiabatic condition (TMRad). We found that the calculation of TMRad based on DSC data could be incorrect when assuming a zero-order kinetic reaction. By using a process temperature of 70 °C, the extrapolated final temperature was found to be 544 °C from DSC experiments, T D24 was estimated to 20 °C based on ARC experiment, and TMRad was calculated to 164 min from ARC experiments. These criteria indicate the process can lead to a thermal runaway. Therefore, we recommend that vegetable oil epoxidation by peroxycarboxylic acid should not be performed in batch reactor, but in semi-batch mode.












Similar content being viewed by others
References
Anastas PT, Warner JC. Green chemistry: theory and practice. Oxford: Oxford University Press; 2000.
Noyori R. Pursuing practical elegance in chemical synthesis. Chem Commun. 2005;14:1807–11.
Meier MAR, Metzger JO, Schubert US. Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev. 2007;36:1788.
Xia Y, Larock RC. Vegetable oil-based polymeric materials: synthesis, properties, and applications. Green Chem. 2010;12:1893–909.
Hernández N, Williams RC, Cochran EW. The battle for the “green” polymer. Different approaches for biopolymer synthesis: bioadvantaged vs. bioreplacement. Org Biomol Chem. 2014;12:2834–49.
Köckritz A, Martin A. Oxidation of unsaturated fatty acid derivatives and vegetable oils. Eur J Lipid Sci Technol. 2008;110:812–24.
Di Serio M, Turco R, Pernice P, Aronne A, Sannino F, Santacesaria E. Valuation of Nb2O5–SiO2 catalysts in soybean oil epoxidation. Catal Today. 2012;192:112–6.
Arends IWCE, Sheldon RA. Recent developments in selective catalytic epoxidations with H2O2. Top Catal. 2002;19:133–41.
Bunton CA, Lewis TA, Llewelyn DL. Tracer studies in the formation and reactions of organic per-acids. J Am Chem Soc. 1956;78(6):1226–30.
Shi H, Zhang Z, Wang Y. Mechanism on epoxidation of alkenes by peracids: a protonation-promoted pathway and its quantum chemical elucidation. J Mol Catal A Chem. 2005;238:13–25.
Sinadinović-Fišer S, Janković M, Borota O. Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chem Eng Proc. 2012;62:106–13.
Turco T. Industrial catalytic processes intensification through the use of microreactors. PhD dissertation, The University of Naples Federico II, Naples, 2010.
Leveneur S, Wärnå J, Salmi T, Murzin DY. A review: catalytic synthesis and decomposition of peroxycarboxylic acids. Trends Chem Eng. 2010;13:17–52.
Leveneur S, Thönes M, Hébert J-P, Taouk B, Salmi T. From kinetic study to thermal safety assessment: application to peroxyformic acid synthesis. Ind Eng Chem Res. 2012;51(13999):14007.
Stoessel F. Thermal safety of chemical processes. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2008.
Sun D-X, Miao X, Xie C-X, Gu J, Li R. Study on thermal properties and kinetics of benzoyl peroxide by ARC and C80 methods. J Therm Anal Calorim. 2012;107:943–8.
Wu S-H, Chou H-C, Pan R-N, Huang Y-H, Horng J-J, Chi J-H, Shu C-M. Thermal hazard analyses of organic peroxides and inorganic peroxides by calorimetric approaches. J Therm Anal Calorim. 2012;109:355–64.
Cheng S-Y, Tseng J-M, Lin S-Y, Gupta JP, Shu C-M. Runaway reaction on tert-butyl peroxybenzoate by DSC tests. J Therm Anal Calorim. 2008;93:121–6.
Casson V, Maschio G. Screening analysis for hazard assessment of peroxides decomposition. Ind Eng Chem Res. 2012;51:7526–35.
Wu S-H, Chi J-H, Huang C-C, Lin N-K, Peng J-J, Shu C-M. Thermal hazard analyses and incompatible reaction evaluation of hydrogen peroxide by DSC. J Therm Anal Calorim. 2010;102:563–8.
You M-L, Tseng J-M, Liu M-Y, Shu C-M. Runaway reaction of lauroyl peroxide with nitric acid by DSC. J Therm Anal Calorim. 2010;102:535–9.
Chi J-H, Wu S-H, Charpentier J-C, Yet-Pole I, Shu C-M. Thermal hazard accident investigation of hydrogen peroxide mixing with propanone employing calorimetric approaches. J Loss Prev Process Ind. 2012;25:142–7.
Salzano E, Agreda AG, Russo V, Di Serio M, Santacesaria E. Safety criteria for the epoxydation of soybean oil in fed- batch reactor. Chem Eng Trans. 2012;. doi:10.3303/CET1226007.
Leveneur S, Delannoy F, Levesqueau Y, Hebert J-P, Estel L, Taouk B, Salmi T. The limit of DSC as a preliminary tool to determine the safety parameters? Chem Eng Trans. 2014;. doi:10.3303/CET1436024.
Keller A, Stark D, Fierz H, Heinzle E, Hungerbühler K. Estimation of the time to maximum rate using dynamic DSC experiments. J Loss Prev Process Ind. 1997;10:31–41.
Blaine RL, Kissinger HE. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6.
Musakka N, Salmi T, Wärnå J, Ahlkvist J, Piironen M. Modelling of organic liquid-phase decomposition reactions through gas-phase product analysis: model systems and peracetic acid. Chem Eng Sci. 2006;61:6918–28.
Leveneur S, Salmi T, Musakka N, Wärnå J. Kinetic study of decomposition of peroxypropionic acid in liquid phase through direct analysis of decomposition products in gas phase. Chem Eng Sci. 2007;62:5007–12.
Acknowledgements
This work was supported by the Academy of Finland and the European Regional Development Fund (INTERREG IVA Grant Number 4274). The authors are particularly grateful to Jean-Pierre Hébert for his contribution to the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leveneur, S., Estel, L. & Crua, C. Thermal risk assessment of vegetable oil epoxidation. J Therm Anal Calorim 122, 795–804 (2015). https://doi.org/10.1007/s10973-015-4793-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4793-8