Abstract
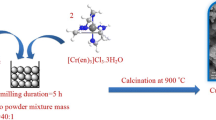
In this work, Cr–urea complex ([Cr(NH2CONH2)6](NO3)3) was synthesized by direct solid-state reaction of chromium nitrate and urea, and its thermal decomposition reaction was studied for the first time to explore the possibilities of using the complex as precursor to nanosized chromium oxide. The formation of [Cr(NH2CONH2)6](NO3)3 is confirmed from infrared spectroscopy and elemental analysis. Thermogravimetric and differential thermal analysis of the compound show a three-stage thermal decomposition in the temperature range from 190 to 430 °C. The result of X-ray diffraction (XRD) shows that the [Cr(NH2CONH2)6](NO3)3 decompose at ~300 °C into α-Cr2O3 nanopowder with an average crystallite size of 33 nm.



Similar content being viewed by others
References
Qiu Y, Gao L. Metal–urea complex—A precursor to metal nitrides. J Am Ceram Soc. 2004;87(3):352–7.
Sardar K, Dan M, Schwenzera B, Rao CNR. A simple single-source precursor route to the nanostructures of AlN GaN and InN. J Mater Chem. 2005;15:2175–7.
Zhang Z, Liu R, Qian Y. Synthesis of nanocrystalline chromium nitride from ammonolysis of chromium chloride. Mater Res Bull. 2002;37:1005–10.
Ren R, Yang Z, Shaw LL. Synthesis of nanostructured chromium nitrides through mechanical activation process. Nanostruct Mater. 1999;11:25–35.
Asuha S, Zhao S, Wu HY, Song L, Tegus O. One step synthesis of maghemite nanoparticles by direct thermal decomposition of Fe–urea complex and their properties. J Alloy Compd. 2009;472:L23–5.
Asuha S, Suyala B, Siqintana X, Zhao S. Direct synthesis of Fe3O4 nanopowder by thermal decomposition of Fe–urea complex and its properties. J Alloy Compd. 2011;509:2870–3.
Ravindran B, Madhurambal G, Mariappan M, Mojumdar SC. Synthesis and characterization of some single crystals of thiourea urea zinc chloride. J Therm Anal Calorim. 2011;104:893–9.
Yurdakul H, Turan S, Ozel E. The mechanism for the colour change of iron chromium black pigments in glazes through transmission electron microscopy techniques. Dyes Pigments. 2011;91:126–33.
Abu-Zied BH. Structural and catalytic activity studies of silver/chromia catalysts. Appl Catal A. 2000;198:139–53.
Kitsunai H, Hokkirigawa K, Tsumaki N, Kato K. Transitions of microscopic wear mechanism for Cr2O3 ceramic coatings during repeated sliding observed in a scanning electron microscope tribosystem. Wear. 1991;151:279–89.
Vijay R, Sundaresan R, Maiya MP, Srinivasa Murthy S. Hydrogen storage properties of Mg–Cr2O3 nanocomposites: the role of catalyst distribution and grain size. J Alloy Compd. 2006;424:289–93.
Hou X, Choy KL. Synthesis of Cr2O3–based nanocomposite coatings with incorporation of inorganic fullerene-like nanoparticles. Thin Solid Films. 2008;51:8620–4.
Cantalini C. Cr2O3, WO3 single and Cr/W binary oxide prepared by physical methods for gas sensing applications. J Eur Ceram Soc. 2004;24:142–4.
Yang J, Tao Q, Frost RL, Kristóf J, Horváth E. Studies on self-assembly hydrothermal fabrication and thermal stability of chromium oxyhydroxide nanomaterials synthesised from chromium oxide colloids. J Therm Anal Calorim. 2013;111:329–34.
Penland RB, Mizushima S, Curran C, Quagliano JV. Infrared absorption spectra of inorganic coordination complexes X. Studies of some metal–urea complexes. J Am Chem Soc. 1957;79:1575–8.
Lima MD, Bonadimann R, de Andrade MJ, Toniolo JC, Bergmann CP. Nanocrystalline Cr2O3 and amorphous CrO3 produced by solution combustion synthesis. J Eur Ceram Soc. 2006;26:1213–20.
Schaber PM, Colson J, Higgins S, Thielen D, Anspach B, Brauer J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim Acta. 2004;424:131–42.
Biamino S, Badini C. Combustion synthesis of lanthanum chromite starting from water solutions: investigation of process mechanism by DTA–TGA–MS. J Eur Ceram Soc. 2004;24:3021–34.
Melnikov P, Nascimento VA, Arkhangelsky IV, Zanoni Consolo LZ, de Oliveira LCS. Thermolysis mechanism of chromium nitrate nonahydrate and computerized modeling of intermediate products. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-013-3106-3.
Aghaie-Khafri M, Kakaei Lafdani MH. A novel method to synthesize Cr2O3 nanopowders using EDTA as a chelating agent. Powder Technol. 2012;222:152–9.
Kim DW, Shin SI, Lee JD, Oh SG. Preparation of chromia nanoparticles by precipitation–gelation reaction. Mater Lett. 2004;58:1894–8.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21267016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, M.L., Zhao, S. & Asuha, S. Synthesis and thermal decomposition of Cr–urea complex. J Therm Anal Calorim 115, 255–258 (2014). https://doi.org/10.1007/s10973-013-3260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3260-7