Abstract
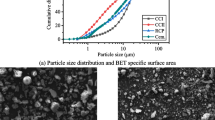
Three sodium waterglass (NWG) such as commercial NWG (S1), NWG from pure rice husk ash (S2) and NWG from raw rice husk ash (S3) were applied for producing geopolymer cements using metakaolin (MK) as aluminosilicate source. Geopolymers (Geo1, Geo2 and Geo3) were prepared using each NWG with the molar ratios SiO2/Na2O and H2O/Na2O kept constant at 1.5 and 12, respectively. It could be observed that the water absorption of Geo1, Geo2 and Geo3 is 7, 9 and 13.2 % and the mass loss is 15.8, 14.7 and 12.4 %, respectively. Their compressive strength at 20 days (37.5/34.3/29.6 MPa) and 28 days (43.3/40.3/33.2 MPa) increases with increasing the aging and decreases in the course Geo1/Geo2/Geo3. Their average pore radius (6/8/20 nm) and cumulative pore volumes (155/205/245 mm3/g) increase in the course Geo1/Geo2/Geo3. It is discussed that the presence of phosphate known as corrosion inhibitors in raw rice husk ash hinders the dissolution of SiO2. It entails the formation of NaH2PO4 in S3 which reduces the soluble Si atoms. Therefore, less amount of metakaolin could be dissolved leaving thus a higher amount of unreacted metakaolin particles in Geo3. The reacted volumes and compositions of the geopolymers are different in the three cases, too. A content of approximately 20, 25 and 35 % of unreacted metakaolin was proved for Geo1, Geo2 and Geo3, respectively.
Graphical Abstract















Similar content being viewed by others
References
Davidovits J (1991) Geopolymers: inorganic polymeric new materials. J Therm Anal Calorim 37:1633–1656
Turner LK, Collins FG (2013) Carbon dioxide equivalent (CO2-e) emissions: a comparison between geopolymer and OPC cement concrete. Constr Build Mater 43:125–130
Brykov AS, Korneev VI (2008) Production and usage of powdered alkali metal silicate hydrates. Metallurgist 52:648–652
Rozainee M, Ngo SP, Salema AA (2008) Effect of fluidising velocity on the combustion of rice husk in a bench-scale fluidised bed combustor for the production of amorphous rice husk ash. Bioresour Technol 99:703–713
Kumar A, Mohanta K, Kumar D, Parkash O (2012) Properties and industrial applications of rice husk: a review. Inter J Emerg Technol Adv Eng 2:86–90
Tchakouté HK, Rüscher CH, Kong S, Ranjbar N (2015) Synthesis of sodium waterglass from rice husk ash as activator to produce metakaolin-based geopolymer cements. J Build Eng (submitted)
Rüscher CH, Mielcarek EM, Wongpa J, Jaturapitakkul C, Jarasit F, Lohaus L (2011) Silicate-aluminosilicate and calcium silicate gels for building materials: chemical and mechanical properties during ageing. Eur J Mineral 23:111–124
Balczár I, Korim T, Dobrádi A (2015) Correlation of strength to apparent porosity of geopolymers—understanding through variations of setting time. Constr Build Mater 93:983–988
ASTM C642-06 (2006) Standard test method for density, absorption, and voids in hardened concrete. ASTM International, United States
Kakali G, Perraki T, Tsivilis S, Badogiannis E (2001) Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Appl Clay Sci 20:73–80
Nayak PS, Singh BK (2007) Instrumental characterization of clay by XRF, XRD and FTIR. Bull Mater Sci 30:235–238
Vizcayno C, de Gutierrez RM, Castello R, Rodriguez E, Guerrero CE (2010) Pozzolan obtained by mechanochemical and thermal treatments of kaolin. Appl Clay Sci 49:405–413
Tchakouté HK, Rüscher CH, Djobo JNY, Kenne BBD, Njopwouo D (2015) Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl Clay Sci 107:188–194
Halasz I, Agarwal M, Li R, Miller N (2007) Vibrational spectra and dissociation of aqueous Na2SiO3 solutions. Catal Lett 117:34–42
Halasz I, Agarwal M, Li R, Miller N (2010) What can vibrational spectroscopy tell about the structure of dissolved sodium silicates? Microporous Mesoporous Mater 135:74–81
Gaggiano R, De Graeve I, Mol JMC, Verbeken K, Kestens LAI, Terryn H (2013) An infrared spectroscopic study of sodium silicate adsorption on porous anodic alumina. Surf Interface Anal 45:1098–1104
Langille KB, Nguyen D, Bernt JO, Veinot DE, Murthy MK (1991) Mechanism of dehydration and intumescence of soluble silicates: part I effect of silica to metal oxide molar ratio. J Mater Sci 26:695–703
Autef A, Prud’Homme E, Joussein E, Gasgnier G, Pronier S, Rossignol S (2013) Evidence of a gel in geopolymer compounds from pure metakaolin. J Sol-Gel Sci Technol 67:534–544
Gharzouni A, Joussein E, Samet B, Baklouti S, Rossignol S (2015) Effect of the reactivity of alkaline solution and metakaolin on geopolymer formation. J Non-Cryst Solids 410:127–134
Dimas D, Giannopoulou I, Panias D (2009) Polymerization in sodium silicate solutions: a fundamental process in geopolymerization technology. J Mater Sci 44:3719–3730
Galan E, Aparicio P, Miras A, Michailidis K, Tsirambides A (1996) Technical properties of compounded kaolin sample from Griva (Macedonia, Greece). Appl Clay Sci 10:477–490
Yohai L, Vázquez M, Valcarce MB (2013) Phosphate ions as corrosion inhibitors for reinforcement steel in chloride-rich environments. Electrochim Acta 102:88–96
Rüscher CH, Mielcarek E, Lutz W, Ritzmann A, Kriven WM (2010) Weakening of alkali-activated metakaolin during aging investigated by the molybdate method and infrared absorption spectroscopy. J Am Ceram Soc 93:2585–2590
Kamseu E, Nait-Ali B, Bignozzi MC, Leonelli C, Rossignol S, Smith DS (2012) Bulk composition and microstructure dependence of effective thermal conductivity of porous inorganic polymer cements. J Eur Ceram Soc 32(8):1593–1603
Kamseu E, Zénabou NM, Gouloure N, Nait-Ali B, Zekeng S, Melo UC, Rossignol S, Leonelli C (2015) Cumulative pore volume, pore size distribution and phases percolation in porous inorganic polymer composites: relation microstructure and effective thermal conductivity. Energy Build 88:45–56
Duxson P, Provis JL, Lukey GC, Mallicoat SW, Kriven WM, Van Deventer JSJ (2005) Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf A Physicochem Eng Asp 269:47–58
Khale D, Chaudhary R (2007) Mechanism of geopolymerization and factors influencing its development: a review. J Mater Sci 42:729–746
Milkey RG (1960) Infrared spectra of some tectosilicates. Am Miner 45:990–1007
Davidovits J (2011) Geopolymer chemistry and applications, 3rd edn. Institute Geopolymer, Saint-Quentin, pp 356–357
Gao K, Lin KL, Wang D, Hwang CL, Shiu HS, Chang YM, Cheng TW (2014) Effect of SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr Build Mater 53:503–510
Lecomte I, Henrist C, Liegeois M, Maseri F, Rulmont A, Cloots R (2006) (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J Eur Ceram Soc 26:3789–3797
Rosas-Casarez CA, Arredondo-Rea SP, Gómez-Soberón JM, Alamaral-Sánchez JL, Corral-Higuera R, Chinchillas-Chinchillas MJ, Acuña-Agüero OH (2014) Experimental study of XRD, FTIR and TGA techniques in geopolymeric materials. In: Proceedings of the international conference on advances in civil and structural engineering-CSE 2014. doi:10.15224/978-1-63248-006-4-65
Duxson P, Lukey GC, van Deventer JSJ (2007) Physical evolution of Na-geopolymer derived from metakaolin up to 1000°C. J Mater Sci 42:3044–3054
Temuujin J, Minjigmaa A, Rickard W, van Riessen A (2012) Thermal properties of spray-coated geopolymer-type compositions. J Therm Anal Calorim 107:287–292
Ramadhansyah PJ, Mahyun AW, Salwa MZM, Abu Bakar BH, Megat Johari MA, Wan Ibrahim MH (2012) Thermal analysis and pozzolanic index of rice husk ash at different grinding time. Procedia Eng 50:101–109
Acknowledgments
Hervé Tchakouté Kouamo gratefully acknowledges the Alexander von Humboldt Foundation for financially support his Postdoctoral research (No. KAM/1155741 STP) in Institut für Mineralogie, Leibniz Universität Hannover, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tchakouté, H.K., Rüscher, C.H., Kong, S. et al. Comparison of metakaolin-based geopolymer cements from commercial sodium waterglass and sodium waterglass from rice husk ash. J Sol-Gel Sci Technol 78, 492–506 (2016). https://doi.org/10.1007/s10971-016-3983-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-3983-6