Abstract
Hydrothermal and reflux synthesis methods were utilized for the preparation of 5% Sn doped TiO2 nanopowders. Obtained powders were subjected to particle size and specific surface area measurements, flame atomic absorption spectroscopy, X-ray diffraction analyses and transmission electron microscopy examination. TiO2 powder prepared by hydrothermal synthesis presented a bimodal particle size distribution with average particle sizes of about 3 and 10 nm, whereas TiO2 powder synthesized by reflux method had an average particle size of 7 nm. Specific surface areas of hydrothermal and reflux synthesized TiO2 were 130 and 115 m2/g, respectively. Sn doping in TiO2 was 95.8% in hydrothermal and 86.4% in reflux synthesis as measured by FAAS, respectively. Photocatalytic activities of the obtained TiO2 powders were evaluated by decomposition of malachite green solution in a solar box. Photocatalytic activity of hydrothermally synthesized TiO2 was fairly higher in visible light and slightly higher in UV light than the activity of reflux synthesized TiO2.
Similar content being viewed by others
1 Introduction
TiO2 powder in nanometric form has been widely utilized as a pigment, as an additive in paints and in ointments, and in photovoltaic and electrochromic applications. It has also been used in photocatalytic sterilization, self-cleaning (SC), organic contaminant treatment and air purification applications due to its well-known photocatalytic effect [1–5]. Photocatalysis is essentially the breakdown of organic pollutant compounds on the surface of TiO2, with the aid of ultraviolet (UV) or visible (VIS) light [1–3, 6]. The SC property of TiO2 in both powder and film form has been widely investigated [1, 2, 4, 5, 7, 8], however, these studies mostly dealt with TiO2 powder [2, 7, 8]. The efficiency of photocatalysts in powder form is superior to films due to the much larger specific surface area of powders, which provides relatively shorter duration of photocatalytic cleaning reactions.
Removal of organic contaminants in wastewater is an important issue for preventing the pollution of environment and preserving the clean water sources. Dyes, arising especially from textile industry are toxic pollutants that are present in industrial wastewater. Their treatment via physical or biological means is difficult. Utilization of TiO2 as a promising heterogeneous photocatalyst for removal of organic pollutants from industrial wastewater has emerged in the last decade.
Many oxides have suitable band gap for photocatalysis. Among them, TiO2 has attracted attention since it encompasses better properties such as low cost, non-toxicity and chemically stability. One significant downside of TiO2 for SC applications is its wide band gap (Eg = 3.2 eV), which bounds its service to UV irradiation only. Thus, most of the solar irradiation (∼95%) cannot be benefited by the TiO2 photocatalyst. Metal ion doping into TiO2 has been investigated for providing absorbance also in the visible region. Another aid of doping TiO2 with metal ions in proper amounts, was suggested to be suppressing of the hole–electron recombination. Electron- hole recombination is detrimental for photocatalytic activity and it decreases efficiency of photocatalyst [9, 10].
Photocatalytic activity of TiO2, doped with metal ions such as Al3+, V5+, Cr3+, Mn2+, Fe3+, Zn2+, Sn4+, and Ce4+, has been investigated [5, 11–14]. Type and amount of doping is important in enhancing the photoactivity of TiO2 under UV and visible light. Dopants, in specific ranges depending on the type of the dopant, were reported to be effective in augmenting the photocatalytic activity of TiO2. On the other hand, ratios of dopants out of the proper range were harmful [11, 13, 15, 16]. It is difficult to compare the activities or ratios of dopants in TiO2 reported in different studies in literature, since TiO2 was prepared by various methods and doping contents were reported in various forms like mole %, wt. %, or wt. % of dopant compound for instance in the form of metal nitrate, etc. [17].
Photoactivity of Sn doped TiO2 powder has been investigated [12–15, 18], and 5% Sn was reported to enhance the activity of TiO2 [12, 13]. The positive effects of SnO2 and also of other dopants was proposed to be introducing new electronic states into the band gap of TiO2, decreasing the TiO2 particle size and increasing the surface area thereby providing higher adsorption, and separation of the charge carriers [14, 15, 18].
TiO2 powders, doped with different metal ions were prepared via various techniques such as sol–gel [11–14], hydrolysis of inorganic salts [19], ultrasonic technique [20] and hydrothermal method [13, 21–23]. The extent of doping depends on the type of the synthesis method. In most of the processes, a calcinations step is required in order to attain the crystal structure of TiO2. Calcination results in growth of TiO2 grains, leading to loss of surface area and loss of photocatalytic efficiency [21]. Hydrothermal process is a relatively low temperature technique, in which the reaction takes place in a closed autoclave under high vapor pressure. Good control of the composition of the products is achieved in hydrothermal process and calcination of the products is mostly not necessary. It is environmentally friendly since reactions proceed in a closed system [21, 22, 24]. The need for an expensive autoclave may be counted as the drawback of this process. Reflux synthesis is also a low temperature method. In this method the reaction takes place in a heated container, which is connected to a reflux condenser system open to atmosphere. The vaporized solvents are condensed back into the reaction medium and long lasting reactions can be accomplished.
Aim of this study is to prepare Sn doped TiO2 nanopowders by two different techniques, namely reflux and hydrothermal methods, and to investigate the photocatalytic activity of the formed powders. These methods were selected due to their low process temperature and lack of necessity for calcination of the products. For this purpose, photocatalytic degradation of malachite green (MG), a dye that is commonly used in textile industry, was tested in the presence of prepared TiO2 powders by two different methods.
2 Materials and methods
2.1 Chemicals and apparatus
The reagents employed were titanium (IV)-n-butoxide [Ti(OBun)4, 97%, Fluka] as TiO2 source, hydrochloric acid (Merck, 37%) as catalyst, tin (IV) chloride (Alfa Aesar, 98%) as dopant, deionized water as hydrolysis agent. Malachite Green (MG) was used as a model pollutant (analytical reagent grade).
Nano-TiO2 powders were synthesized by two different methods. In the first method, a Berghoff hydrothermal unit having time and temperature controllers was utilized. In the second method a condenser reflux system was used. A Rigaku Geigerflex D Max/B model X-ray diffractometer (XRD) with Cu Kα radiation (λ = 0.15418 nm) in the region 2θ = 10°–70° with a step size of 0.04° was used for determining the crystalline phases.
Size of Sn doped TiO2 particles was investigated by transmission electron microscopy (TEM, FEI Tecnai G2F30). Elemental composition of the particles was investigated by flame atomic absorption spectroscopy (FAAS).
Dye concentration in the aqueous solution was measured in predetermined durations after irradiation by a Varian Carry 5000 model UV–vis–NIR spectrophotometer. Sn doped TiO2/dye solution was irradiated with/without 400 nm cut-off filter in a solar box (Erichsen, Model 1500) with an Xe lamp (690 W/m2).
2.2 TiO2 powder synthesis
Flowsheets for hydrothermal and reflux syntheses are presented in Scheme 1.
2.2.1 Hydrothermal method
Ti(OBun)4 was cooled in an ice-bath, then HCl (37% w/w) was added within 15 min into the cooled Ti(OBun)4 dropwise by a burette. After stirring for 5 min at ambient temperature, required amount of tin (IV) chloride was added. Final solution was stirred until a clear and homogeneous solution was formed. Then, required amount of water was added within 10 min into the solution dropwise by a burette. HCl/Ti(OBun)4, SnCl4/Ti(OBun)4 and H2O/Ti(OBun)4 mol/mol ratios were 0.296, 0.05 and 2.06, respectively. Gelation occurred after water addition. Reaction was allowed for 2 h, and then a viscose solution was obtained. The solution was transferred into a 250 mL Teflon bomb which was then closed. The closed bomb was placed inside a preheated (200 °C) stainless steel autoclave. Reaction was conducted at 200 °C for 1 h. The Teflon bomb was removed from the hydrothermal unit and it was cooled to room temperature. Obtained powders were separated by centrifuging and they were dried in a vacuum sterilizer. Thus, nanosized TiO2 particles having yellow color were obtained.
2.2.2 Reflux method
Nano-TiO2 particles were synthesized with the same molar ratios as mentioned above. The reaction was carried out in a glass balloon which was heated in an oil bath at 130 °C for 5 h. The balloon was connected to a reflux condenser system open to atmosphere.
2.3 Preparation of TiO2 sols
TiO2 (0.625 g) sols were prepared by ultrasonically dispersing the synthesized TiO2 powder in deionized water (for H-SnTiO2), and in water: n-propanol (for R-SnTiO2) (1:1, w/w) mixture without using a dispersant. The solvents and TiO2 particles were treated in an ultrasonic bath for a few minutes. Finally, transparent TiO2 sols were obtained.
2.4 Photocatalytic degradation of malachite green
For photocatalytic decomposition experiments, 62.5 μL of dye solution (from 2.5 ppm stock solution of MG) was added into TiO2 sol (24.94 mL, 2.5% TiO2 (w/w)). The light of the empty solar box was kept on for 15 min for stabilization before starting the test. Meanwhile, the nano-TiO2/MG sol was poured into the transparent polysytrene reaction cell consisting of 12 separate compartments. The cell was placed into the Solar Box. The decomposition of MG was monitored by measuring the absorbance at 617 nm (λ max) by UV–VIS spectrophotometer. Decomposition of MG was quantified by detecting the MG concentration after 20, 40 and 60 min during UV and VIS—irradiation.
3 Result and discussions
3.1 Structure of TiO2 powders
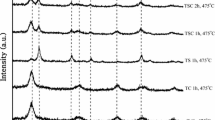
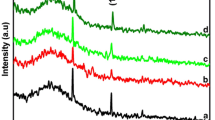
XRD patterns of Sn doped TiO2 powders prepared by hydrothermal and reflux methods are presented in Fig. 1. It was seen that TiO2 powders synthesized by both methods had anatase crystal structure. All the peaks of anatase phase TiO2 could be clearly determined on the XRD patterns of the synthesized powders. Peaks of rutile and brookite phases, which are known to have less photocatalytic effect, were not present. The peaks on the XRD patterns at 25.39°, 38.11°, 48.47° and 55.01° pertaining to (101), (004), (200) and (211) planes matched well with those of anatase TiO2 reported in literature [25] (ICDD card no: 21-1272). The peaks were seen to be wide, which can be taken as an indication of the nanometric size of the TiO2 crystallites.
Particle size distributions of the synthesized TiO2 powders which were determined by a Malvern Zetasizer Z/S unit are presented in Fig. 2. TiO2 powder which was synthesized by reflux method had an average particle size of 7 nm. On the other hand, hydrothermally synthesized TiO2 presented a bimodal particle size distribution with average particle sizes of about 3 and 10 nm. Formation of larger particles in hydrothermal synthesis as compared to reflux method may be due to higher temperature utilized in this process. Hydrothermal synthesis was conducted at 200 °C, whereas in reflux method temperature was kept at 130 °C. Surface areas of the Sn doped TiO2 powders synthesized by hydrothermal and reflux methods were 130 and 115 m2/g, respectively. Comparison of the particle size and specific surface area of TiO2 powders is presented in Table 1. Photocatalytic activity of nano TiO2 powder is strongly related to particle size and specific surface area. The lower is the particle size and the higher is the specific surface area, the higher is the photocatalytic effect. In the employed methods, crystalline anatase phase TiO2 was obtained at low temperatures and as a result the calcination step was avoided. This helped preserving the nanostructure of the TiO2 particles. Therefore, the obtained powders in both methods presented high specific surface areas.
TEM micrographs the TiO2 powders which were obtained by hydrothermal and reflux methods are presented in Fig. 3a, b c, d respectively. In the presented TEM micrographs sizes of the individual particles can be visualized to be in nanometric scale. The particle sizes observed in the TEM micrographs are in accord with the particle sizes which were measured by Zetasizer Nano Z/S as presented in Fig. 2. TiO2 particles are about 5 nm as shown in Fig. 3.
The molar ratio of Sn to Ti was kept at 5–95 as shown in Scheme 1. This amount of Sn corresponds to 4.71 mol % (or 6.83 wt%) in the total amount of synthesized Sn doped TiO2 powders. The filtrate solutions after reflux synthesis and hydrothermal synthesis were analyzed by FAAS and it was determined that formed TiO2 contained 5.9 wt% Sn and 6.54 wt% Sn, respectively. These results indicate that 86.38% of the Sn in reflux synthesis and 95.75% of Sn in hydrothermal synthesis were incorporated into the TiO2 lattice structure. Higher doping amount of Sn into TiO2 in hydrothermal synthesis may be due to higher temperature applied in this method and also may be due to high pressure provided in the autoclave.
3.2 Photocatalytic activity tests
Photocatalytic effects of Sn doped TiO2 powders obtained by the two methods were investigated by keeping 2.5 ppm MG in 2.5% (w/w) TiO2 sol for 20–60 min under UV and VIS light in a solar box and by measuring the absorbance of MG by spectrophotometer. Height of the MG peak at 617 nm corresponds to the concentration of MG in solution. Height of the peak of MG was measured and thus % decomposition of MG as a result of photocatalytic reaction was determined by following the decrease of the peak height. The results are presented in Fig. 4. Photocatalytic activity of hydrothermally synthesized TiO2 was seen to be considerably higher than that of reflux synthesized TiO2. This was an expected result since Sn doping achieved in hydrothermally synthesized TiO2 was higher than that in reflux synthesized TiO2, as shown in Table 1 and Fig. 4. Sn doping causes a shift in the absorbance of the TiO2 powders into visible region [22]. Higher Sn dopant amount leads to larger absorbance, which results in higher photocatalytic activity under visible light. In addition, specific surface area of H-SnTiO2 was larger than that of R-SnTiO2. This is another reason of higher photocatalytic activity of H-SnTiO2. On the other hand in the UV illumination, photocatalytic activity of H-SnTiO2 was slightly higher than or similar to that of R-SnTiO2 as shown in Fig. 4. Slightly higher photocatalytic activity of H-SnTiO2 may be a consequence of its larger surface area.
It is difficult to compare the obtained results with those of the previous studies since in the literature decomposition of different organic agents at different concentrations was investigated. In the study of Liqiang et al. [26], Sn doped TiO2 nano powder was obtained through sol–gel route by mixing Ti(OBu)4, ethanol, H2O and HNO3. Products were calcined at 500–700 °C. Photocatalytic degradation of phenol was the highest (20% degradation in 1 h) when the powder was calcined at 600 °C. In another study, Sn doped TiO2 was utilized in the form of films on glass surfaces by Sayılkan et al. [27]. MG degradation of the obtained films were much lower when compared to that of the Sn doped TiO2 powders in the present study. In 1 h, degradation of MG was reported to be less than 50%. This is an expected result since the surface area of the film is lower than that of TiO2 in powder form.
4 Conclusion
Synthesis of 5 mol % Sn doped TiO2 (Sn to Ti molar ratio 5/95) nanopowders were accomplished through hydrothermal and reflux methods at 200 and 130 °C, respectively. Both methods produced anatase phase TiO2 at low temperatures therefore calcination of obtained powders was not necessary. Hydrothermally synthesized TiO2 powder presented higher photocatalytic activity than that of reflux synthesized TiO2. This was attributed to higher Sn content as well as larger specific surface area of hydrothermal TiO2. Reflux method is advantageous in terms of lower equipment cost and less labor over hydrothermal synthesis. Further adjustment of experimental parameters of reflux synthesis such as lower temperature and longer duration may result in formation of TiO2 powders having higher Sn content and higher specific surface area. Hazardous effects of organic contaminants to the environment can be prevented by the use of photocatalytic TiO2 powder prepared by hydrothermal or reflux synthesis.
References
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Fujishima A, Zhang X, Tryk DA (2008) TiO2 photocatalysis and relate surface phenomena. Surf Sci Rep 63:515–582
Lasa H, Serrano B, Salaces M (2005) Photocatalytic reaction engineering. Springer Science+Business Media Inc, New York
Kesmez Ö, Çamurlu HE, Burunkaya E, Arpaç E (2009) Sol–gel preparation and characterization of anti-reflective and self-cleaning SiO2–TiO2 double-layer nanometric films. Sol Energy Mater Sol Cells 93:1833–1839
Burunkaya E, Kesmez Ö, Kiraz N, Çamurlu HE, Asiltürk M, Arpaç E (2010) Sn4+ or Ce3+ doped TiO2 photocatalytic nanometric films on antireflective nano-SiO2 coated glass. Mater Chem Phys 120:272–276
Liu Z, Zhang X, Murakami T, Fujishima A (2008) Sol-gel SiO2/TiO2 bilayer films with self-cleaning and antireflection properties. Sol Energy Mater Sol Cells 92:1434–1438
Wu T, Liu G, Zhao J, Hidaka H, Serpone N (1998) Photoassisted degradation of dye pollutants. V. self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J Phys Chem B 102:5845–5851
Asiltürk M, Sayılkan F, Erdemoğlu S, Akarsu M, Sayılkan H, Erdemoğlu M, Arpaç E (2006) Characterization of the hydrothermally synthesized nano-TiO2 crystallite and the photocatalytic degradation of Rhodamine B. J Hazard Mater B 129:164–170
Bellardita M, Addamo M, Di Paola A, Palmisano L (2007) Photocatalytic behaviour of metal-loaded TiO2 aqueous dispersions and films. Chem Phys 339:94–103
Shi JW, Zheng JT, Wu P (2009) Preparation, characterization and photocatalytic activities of holmium-doped titanium dioxide nanoparticles. J Hazard Mater 161:416–422
Yao M, Chen J, Zhao C, Chen Y (2009) Photocatalytic activities of Ion doped TiO2 thin films when prepared on different substrates. Thin Solid Films 517:5994–5999
Liu B, Zhao X, Zhang N, Zhao Q, He X, Feng J (2005) Photocatalytic mechanism of TiO2–CeO2 films prepared by magnetron sputtering under UV and visible light. Surf Sci 595:203–211
Sayılkan F, Asiltürk M, Tatar P, Kiraz N, Arpaç E, Sayılkan H (2007) Photocatalytic performance of Sn-doped TiO2 nanostructured mono and double layer thin films for Malachite Green dye degradation under UV and vis-lights. J Hazard Mater 144:140–146
Caimei F, Peng X, Yanping S (2006) Preparation of Nano-TiO2 doped with cerium and its photocatalytic activity. J Rare Earth 24:309–313
Stengl V, Bakardjieva S, Murafa N (2009) Preparation and photocatalytic activity of rare earth doped TiO2 nanoparticles. Mater Chem Phys 114:217–226
Wang Z, Chen C, Wu F, Zou B, Zhao M, Wang J, Feng C (2009) Photodegradation of rhodamine B under visible light by bimetal codoped TiO2 nanocrystals. J Hazard Mater 164:615–620
Di Paola A, Garcia-Lopes E, Ikeda S, Marci G, Ohtani B, Palmisano L (2002) Photocatalytic degradation of organic compounds in aqueous systems by transition metal doped polycrystalline TiO2. Catal Today 75:87–93
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Zhang Y, Xiong G, Yao N, Yang W, Fu X (2001) Preparation of titania-based catalysts for formaldehyde photocatalytic oxidation from TiCl4 by the sol–gel method. Catal Today 68:89–95
Wu XM, Wang L, Tan ZC, Li GH, Qu SS (2001) Preparation, characterization and low-temperature heat capacities of nanocrystalline TiO2 ultrafine powder. J Solid State Chem 156:220–224
Akarsu M, Asilturk M, Sayılkan F, Kiraz N, Arpaç E, Sayılkan H (2006) A novel approach to the hydrothermal synthesis of anatase Titania nanoparticles and the photocatalytic degradation of rhodamine B. Turk J Chem 30:333–343
Arpaç E, Sayılkan F, Asiltürk M, Tatar P, Kiraz N, Sayılkan H (2007) Photocatalytic performance of Sn-doped and undoped TiO2 nanostructured thin films under UV and vis-lights. J Hazard Mater 140:69–74
Vigil E, Ayllon JA, Peiro AM, Clemente RR (2001) TiO2 layers grown from flowing precursor solutions using microwave heating. Langmuir 17:891–896
Burunkaya E, Kiraz N, Kesmez Ö, Çamurlu HE, Asiltürk M, Arpaç E (2010) Preparation of aluminum-doped zinc oxide (AZO) nano particles by hydrothermal synthesis. J Sol-Gel Sci Technol 55:171–176
Kartini I, Meredith P, Diniz Da Costa JC, Lu GQ (2004) A novel route to the synthesis of mesoporous Titania with full anatase nanocrystalline domains. J Sol-Gel Sci Technol 31:185–189
Liqiang J, Honggang F, Baiqi W, Dejun W, Baifu X, Shudan L, Jiazhong S (2006) Effects of Sn dopant on the photoinduced charge property and photocatalytic activity of TiO2 nanoparticles. Appl Catal B Environ 62:282–291
Sayılkan F, Asiltürk M, Tatar P, Kiraz N, Şener Ş, Arpaç A, Sayılkan H (2008) Photocatalytic performance of Sn-doped TiO2 nanostructured thin films for photocatalytic degradation of malachite green dye under UV and VIS-lights. Mater Res Bull 43:127–134
Acknowledgments
Authors would like to thank Akdeniz University Research Fund for financial support. Technical and financial support of T.R. Prime Ministry State Planning Organization (Project number: 2005 DPT.120.150) and NANOen are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiraz, N., Burunkaya, E., Kesmez, Ö. et al. Preparation of Sn doped nanometric TiO2 powders by reflux and hydrothermal syntheses and their characterization. J Sol-Gel Sci Technol 59, 381–386 (2011). https://doi.org/10.1007/s10971-011-2515-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2515-7