Abstract
The pure, tin (Sn)-doped, lanthanum (La)-doped and Sn/La co-doped titanium dioxide (TiO2) nanomaterials were synthesized using sol-gel method followed by calcination at the temperature of 360 °C, 450 °C and 600 °C, respectively. The structures of the nanomaterials were characterized by X-ray diffraction (XRD), Thermogravimetric (TG), Differential Thermal Analysis (DTA), Scanning Electron Microscopy (SEM), Energy Dispersive Spectrum (EDS), Transmission Electron Microscopy (TEM), X-ray Photoelectron Spectrum (XPS), Diffuse Reflectance Spectrum (DRS), Photoluminescence Spectrum (PL), Brunauer-Emmett-Teller Measurements (BET), respectively. The photocatalytic property of the photocatalysts under UV light was evaluated through the degradation of Rhodamine B (RhB). The results show that the anatase-rutile phase transition is promoted by Sn-doping while La-doping retards the phase transition. However, La doping plays a major role in the process of phase transformation. The photocatalytic activity of pure TiO2 is affected by annealing temperature remarkably and the optimal annealing temperature is 450 °C. The photocatalytic activity of TiO2 is enhanced significantly by Sn and La doping at three different temperatures. Sn/La-TiO2 exhibits the highest degradation rates and the fastest reaction rates probably owing to the synergistic effect of Sn4+ and La3+ ions in inhibiting the recombination of photogenerated electron-hole pairs. The formation of extra surface hydroxyl groups and additional surface area are also beneficial for the photocatalytic activity.
Similar content being viewed by others
Introduction
Environmental pollution is an increasingly serious problem, which restricts the development of economy severely. Photocatalytic technology is an effective way to solve the problem and several semiconductor photocatalysts have been used for decomposing organic pollutants such as zinc oxide1, cadmium sulfide2, carbon nanoparticle3, tungsten trioxide4 and so on. Among numerous candidate compounds, TiO2 has been widely used owing to its high photocatalytic activity, non-toxicity, low cost and stable chemical property5,6,7,8. However, pure TiO2 has two main faults such as low utilization of solar energy because of the wide band gap and low quantum yields since photogenerated electron-hole pairs recombine fast9,10. In order to improve the photocatalytic activity of pure TiO2, researchers adopt some strategies such as depositing with noble metals11,12,13, combining with other semiconductors14,15,16,17 and doping metal18,19,20 or nonmetal elements21,22.
Doping with La element boosts the photocatalytic activity of TiO2 efficiently because La ions are able to capture photogenerated electrons, prolong the lifetime of photogenerated electron-hole pairs and increase the thermostability of anatase phase which has higher photocatalytic activity than that of rutile phase23,24. Xin et al.25 reported that the discoloration rate of RhB was enhanced by La doping of the TiO2 photoelectrode. TiO2 doping with Sn is also an effective method to enhance photocatalytic activity since the coupling of TiO2 with SnO2 decreases the recombination of photogenerated electron-hole pairs and thus improves charge separation20,26,27. Bhange et al.28 have synthesized a series of Sn-doped TiO2 particles with different concentrations and photocatalytic tests show all the Sn-doped TiO2 have higher photocatalytic activity than that of pure TiO2. It is reasonable to suppose that doping with two dopants will lead to a synergistic effect and increase photocatalytic activity owing to their respective advantages. There are several reports about co-doped TiO2 which have proved that doping with two dopants shows higher photocatalytic activity than the single one23,29,30,31,32,33,34.
In the study of co-doped TiO2, most researchers focus on the concentration of dopants, however, research on the influence of heat treatment temperature is relatively few. It is generally known that heat treatment has a significant impact on the photocatalytic activity of TiO2 because crystalline phase and grain size of TiO2 are affected greatly by annealing temperature. Meanwhile, ion doping will impact phase structure and grain size evidently. The reports have mentioned that anatase-rutile phase transition will be restrained by La doping25 while it is promoted by Sn doping26,28. However, the phase transformation is not quite clear when Sn and La are doped together. Therefore, the purposes of this work was to synthesize Sn/La co-doped TiO2 nanomaterials and investigate their anatase-rutile phase transition as well as photocatalytic activities under UV light at different temperatures. For comparison, Sn-doped, La-doped and pure TiO2 nanomaterials were also prepared and investigated. For simplicity, Sn-doped, La-doped, and Sn/La co-doped TiO2 are labeled as Sn-TiO2, La-TiO2 and Sn/La-TiO2, respectively.
Experiment
Preparation of pure and doped TiO 2 nanomaterials
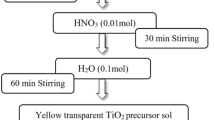
All the TiO2 nanomaterials were synthesized via a sol-gel route. In a typical synthesis of pure TiO2, solution A and B were prepared firstly. Solution A consisted of 15 mL tetrabutyl titanate and 30 mL absolute ethanol. Solution B was made from 5 mL deionized water, 7.5 mL acetic acid and 15 mL absolute ethanol. Solution B was added dropwise to solution A with vigorous stirring. The resulting sol was undergone aging for several hours to form gel. Afterwards, the gel was dried at 80 °C for 12 h and the resulting powders were annealed for 2 h at the temperature of 360 °C, 450 °C and 600 °C, respectively. The Sn-TiO2 and La-TiO2 were synthesized by the same procedure with certain amounts of SnCl4.5H2O or La(NO3)3.6H2O being added into solution B to obtain required doping concentration. The Sn/Ti molar ratios of Sn-TiO2 were 1%, 3%, 6%, 9% and the La/Ti molar ratios of La-TiO2 were 0.2%, 0.5%, 1%, 2%. Sn/La-TiO2 was prepared by the same way and the Sn/Ti, La/Ti molar ratios were 3% and 0.5%, respectively.
Characterization
The crystal structures of the nanomaterials were analyzed using an X-ray diffractometer (XRD, DX-2700, China). Thermogravimetric and differential thermal analysis (TG-DTA) were performed using a thermal analyzer (STA409PC, Germany). Surface morphologies and element compositions were characterized by a field-emission scanning electron microscopy (FEI-Inspect F50, USA) equipped with an energy dispersive X-ray spectrometer (EDS). TEM and HRTEM images were obtained using a Tecnai G2 F20 transmission electron microscope with an acceleration voltage of 200 kV. X-ray photoelectron spectra (XPS) were recorded on a spectrometer (XSAM800, Britain) to examine the chemical states. UV-Vis diffuse reflectance spectra (DRS) were collected on a spectrophotometer (UV-3600, Japan). Photoluminescence (PL) spectra were measured by a luminescence spectrometer (F-4600, Japan) with a 150 W Xenon lamp as an excitation source. Surface areas were tested by using the Brunauer-Emmett-Teller (BET) theory to the nitrogen adsorption-desorption data.
Photocatalytic activity measurement
The photocatalytic activity of the prepared nanomaterials under UV light was evaluated from the degradation of Rhodamine B (RhB). 300 mL 10 mg/L RhB solution was taken into a beaker as an objective degradation pollutant and 0.3 g TiO2 was added as photocatalysts. The mixed liquid was stirring for 30 min in dark to establish an adsorption-desorption equilibrium before illumination. It was then photo-irradiated by using a 250 W high-pressure mercury lamp (main emission wavelength 365 nm, light intensity 35 mW/cm2) as UV light source. The distance between lamp source and beaker was fixed at 10 cm. The change in the concentration of RhB was tested every 30 min by measuring absorbance at the maximum absorptive wavelength (553 nm) using a UV-Vis spectrophotometer (UV6100A, China) and the whole test lasted 180 min. The degradation rate (D) of RhB was calculated by equation: D = (A0 − At)/A0, where A0 and At are the initial absorbance (at 553 nm) and absorbance at time “t”.
Results and Discussion
XRD analysis
Figure 1 shows the XRD patterns of TiO2 nanomaterials at different temperature. All samples show peaks corresponding to anatase phase at the temperature of 360 °C and 450 °C. Compared to 360 °C, the intensity of peaks at 450 °C increases and the width of peaks becomes narrow, which imply that crystalline integrity improves with increase in temperature19,35. Pure TiO2 forms rutile phase when the heat treatment temperature is up to 600 °C. It is clear that the peak intensity of rutile phase of Sn-TiO2 is stronger than that of pure TiO2. However, there is no peak of rutile phase observed in La-TiO2, which indicates that anatase-rutile phase transition is promoted by Sn doping while it is suppressed by La doping. This is in agreement with the literature25,26,28. Ding et al.36 believe that the melting point of doped element oxides will affect anatase-rutile phase transition. The melting point of SnO2 is 1127 °C, which is lower than that of TiO2 (1640 °C), thus Sn as doping element promotes the transition. Meanwhile, the melting point of La2O3 is 2217 °C, which is higher than 1640 °C, as a result, La doping can retard the transition. As depicted in Fig. 1(c), a weak peak which corresponds to rutile (110) plane appears in the pattern of Sn/La-TiO2, indicates that the amount of rutile is less compared to pure TiO2. Obviously, the retarding effect by La doping plays a major role in the process of anatase-rutile phase transition although the dopant concentration of La (0.5%) is less than Sn (3%). The anatase/rutile weight ratios were calculated by the following equation37:
where XR is the weight fraction of rutile, IA and IR are the relative strength of anatase (101) plane and rutile (110) plane, respectively. The average grain size (D) was calculated by Scherer’s formula38:
where λ is the wavelength of Cu Kα used, β is the full with at half maximum of the XRD peak and θ is the Bragg diffraction angle. The results are shown in Table 1. It clearly shows that the grain size of TiO2 decreases by doping and Sn/La-TiO2 presents the smallest grain size at the same temperature.
TG-DTA analysis
Thermal analyses of pure TiO2 (a), Sn-TiO2 (b), La-TiO2 (c) and Sn/La-TiO2 (d) are depicted in Fig. 2. Left side of Y-axis represents the weight loss of samples. Right side of Y-axis represents exothermic and endothermic of samples during the heating process. The weight loss of all samples consists of three steps. In detail, the loss in the first step mainly derives from the evaporation of physically adsorbed water26. The combustion of organic compounds can be responsible for the second weight loss step. The third step of weight loss can be attributed to the dehydroxylation of the gel31. The exothermic peak at 578 °C in the DTA curve of pure TiO2 indicates that the phase transformation from anatase to rutile begin5. For Sn-TiO2 and La-TiO2, the exothermic peaks are approximately at 550 °C and 600 °C, respectively, which make clear that the phase transformation is promoted by Sn doping and is restrained by La doping. This is in accordance with XRD analysis and other literature25,28. It’s remarkable that the exothermic peak of Sn/La-TiO2 is approximately at 590 °C, suggesting that the phase transformation temperature of Sn/La-TiO2 is higher than that of pure TiO2. That means the La doping plays a leading role in the phase transformation process. This result also agrees well with XRD analysis.
SEM and EDS analyses
Figure 3 presents the SEM images of pure TiO2 (a) and Sn/La-TiO2 (b) annealed at 450 °C. It is observed that pure TiO2 consists of agglomerated bulks which show different shapes and sizes. The diameter of aggregates in pure TiO2 ranges from 20–1000 nm. Sn/La-TiO2 presents relative uniform particles distribution and the particles show smaller size. The smaller particle size is favorable for the utilization of light source39.
EDS tests were carried out to examine the element composition of pure and Sn/La-TiO2 annealed at 450 °C and the results are shown in Fig. 3c,d. The peaks of C, Ti, O elements can be observed in the spectrum of pure TiO2. The peak of C derives from conductive plastic which was used in the EDS testing process. Except the peaks of C, Ti, O elements, the peaks of La and Sn elements appear in the spectrum of Sn/La-TiO2, which confirms that La and Sn elements exist in co-doped TiO2 nanomaterials.
TEM analysis
Figure 4 shows the TEM images of (a) pure TiO2 and (b) Sn/La-TiO2. It can be found that the average size of pure TiO2 is about 15 nm. Sn/La-TiO2 presents smaller size compared to pure TiO2, which is in accordance with the XRD results. In order to precisely investigate the structure of pure TiO2 and Sn/La-TiO2, further studies were carried out with a high-resolution transmission electron microscopy (HRTEM) and the images of pure TiO2 and Sn/La-TiO2 are shown in Fig. 4(c,d), respectively. The lattice fringes are clearly observed, which means that both pure TiO2 and Sn/La-TiO2 nanocrystalline form with good crystallinity40. The visible fringe spacings are 0.353 nm in Fig. 4(c) and 0.364 nm in Fig. 4(d), which can be assigned to the (101) plane of anatase TiO2. The (101) plane spacing of Sn/La-TiO2 increases due to the fact that the radius of Sn4+ (0.069 nm) is larger than that of Ti4+ (0.0605 nm), which indicates that Sn4+ ions have entered into TiO2 lattices27.
XPS analysis
In order to confirm the chemical states of elements in pure TiO2 and Sn/La-TiO2 annealed at 450 °C, XPS analysis was further carried out and the results are shown in Fig. 5. Figure 5(a) is the total spectra, which demonstrates that both pure TiO2 and Sn/La-TiO2 contain C, Ti, O elements. Besides, the peak of Sn 3d appears in the pattern of Sn/La-TiO2, indicating that Sn exists in TiO2 sample by doping. The absence of La element is probably because the low content of La is below the limit of XPS detection. The C 1 s peaks are mainly attributed to the oil pollution from equipment.
The high-resolution spectra of Ti 2p of pure TiO2 and Sn/La-TiO2 are shown in Fig. 5(b). It can be clearly observed that Ti 2p of pure TiO2 consists of two peaks at 458.40 eV and 464.09 eV, which correspond to Ti 2p3/2 and Ti 2p1/2, respectively. The slitting between Ti 2p3/2 and Ti 2p1/2 is 5.69 eV, which is convinced that Ti element exists in the form of Ti4+. Similar to pure TiO2, Ti 2p of Sn/La-TiO2 also consists of Ti 2p3/2 and Ti 2p1/2 peaks, which are located at 458.49 eV and 464.11 eV, respectively. The slitting of these two peaks is 5.62 eV, that means Ti element is also +4 state in Sn/La-TiO241,42.
Figure 5(c) shows the high-resolution of Sn 3d spectrum. The peaks at 486.08 eV and 494.80 eV ascribe to Sn 3d5/2 and Sn 3d3/2, respectively, indicating that Sn element exists as Sn4+ 27,28.
The high-resolution spectra of O 1 s of pure TiO2 and Sn/La-TiO2 are shown in Fig. 5(d). The peaks of pure TiO2 at 529.85 eV and 531.83 eV can be attributed to lattice oxygen (O2−) and surface hydroxyl groups (OH−), respectively11,35. Meanwhile, the peaks of lattice oxygen and surface hydroxyl groups for Sn/La-TiO2 are at 530.12 eV and 532.27 eV. It is interesting to note that the proportion of surface hydroxyl groups in Sn/La-TiO2 is 18.1%, which is higher than that of pure TiO2 (16.6%). The extra surface hydroxyl groups are profitable to photocatalytic activity because the surface hydroxyl groups can be transformed into hydroxyl radicals, which decompose dye molecules effectively11.
DRS analysis
Figure 6 shows the DRS spectra of pure TiO2, Sn-TiO2, La-TiO2 and Sn/La-TiO2 calcined at 450 °C. The band gap energy (Eg) of samples can be calculated by the equation:
where α is the absorption coefficient, hν is the photon energy and A is a constant6. The band gap energy of pure TiO2, Sn-TiO2, La-TiO2 and Sn/La-TiO2 are determined to be 3.22 eV, 3.27 eV, 3.35 eV and 3.17 eV, respectively. Sn or La as the doping elements always lead to red shift of TiO2 as reported before27,43, however, in this work, Sn-TiO2 and La-TiO2 show faint blue shift compared to pure TiO2. Bhange et al.28 believe that the blue shift is not owing to the quantum size effect since the grain size of TiO2 is larger than 10 nm, but is the result of the interaction between doping elements and TiO2 lattice and the formation of new energy levels. Sn/La-TiO2 shows slight red shift, which proves that the absorption of light improves by Sn and La co-doping.
PL analysis
It is well known that photoluminescence spectrum results from the recombination of photogenerated electrons and holes29,31,35. Therefore, in order to investigate the recombination and separation of photogenerated pairs, photoluminescence measurement was carried out and the results are shown in Fig. 7. The PL spectra of pure TiO2, Sn-TiO2, La-TiO2 and Sn/La-TiO2 calcined at 450 °C exhibit similar shape, however, the intensities of the samples are different. Pure TiO2 shows the maximal PL intensity compared to doped TiO2, suggesting that the recombination of photogenerated electrons and holes is restrained through Sn or La doping. It is noteworthy that the PL intensity of Sn/La-TiO2 is minimal, which indicates that Sn/La-TiO2 possesses the minimal recombination rate and the maximum separation rate of photogenerated pairs. The higher separation rate is beneficial to photocatalytic activity.
BET analysis
From SEM images results, it is obvious that the aggregation is relieved and the particle size is decreased by Sn and La co-doping. In order to verify that the surface area of TiO2 is increased after co-doping, nitrogen adsorption-desorption isotherm measurement along with BET measurement is carried out and the N2 adsorption-desorption isotherms of pure TiO2 and Sn/La-TiO2 calcined at 450 °C are shown in Fig. 8. The BET specific surface areas of pure TiO2 and Sn/La-TiO2 are determined to be 4.4 m2.g−1 and 85.7 m2.g−1, respectively. The results manifest that Sn/La-TiO2 exhibits higher surface area than pure TiO2. The higher surface area is beneficial for the utilization of light source, which is able to improve photocatalytic activity32.
Photocatalytic activity
The degradation of RhB was employed to evaluate the photocatalytic activity of the prepared TiO2 photocatalysts. Figure 9 shows the photocatalytic activity of Sn-TiO2 and La-TiO2 with different contents annealed at 450 °C.
The adsorption of RhB molecules was investigated via testing the degradation of RhB with catalyst adding under dark condition. The degradation rate of RhB is 3.2% on the condition of pure TiO2 adding in darkness, which is ascribed to the adsorption of RhB molecules on the surface of TiO244. The self degradation of RhB without photocatalyst is 7%, which is negligible in the evaluation. Therefore, the degradation of RhB mainly is derived from the presence of photocatalysts under irradiation.
The degradation rate of pure TiO2 is 82.5% and all of the doped TiO2 show higher degradation rates. The optimal doping concentration for La-TiO2 is 0.5% and for Sn-TiO2 is 3%. Therefore, the molar ratios of Sn/La-TiO2 are determined to be La 0.5% and Sn 3%. The degradation rates of pure TiO2, Sn-TiO2, La-TiO2 and Sn/La-TiO2 annealed at 360 °C, 450 °C and 600 °C are shown in Fig. 10.
The degradation rates of pure TiO2 annealed at 360 °C, 450 °C and 600 °C are 64.7%, 82.5% and 48.0%, respectively. TiO2 annealed at 450 °C possesses the best photocatalytic activity than that of 360 °C and 600 °C. As discussed in XRD analysis, pure TiO2 annealed at 450 °C shows better crystalline integrity of anatase phase compared to 360 °C, which is positive for photocatalytic activity45. Meanwhile, pure TiO2 forms rutile phase at 600 °C and the high rutile content leads to poor photocatalytic activity46,47. Significantly, all of the doped TiO2 photocatalysts exhibit higher photocatalytic performance than pure TiO2 at three temperatures. The degradation rates of RhB for Sn/La-TiO2 are 98.1%, 99.1% and 97.9% at the annealing temperatures of 360 °C, 450 °C and 600 °C, which are 1.52 times, 1.20 times, 2.04 times higher than that of pure TiO2.
From XRD analysis, it can be noted that there is no peak for Sn, La and their oxides. Since the radius of La3+ (0.1061 nm) is much bigger than that of Ti4+ ion (0.0605 nm). Therefore, it is hard for La3+ ions to substitute Ti4+ ions in TiO2 lattice and it is reasonable to suppose that La3+ ions is located at the surface of TiO223,24,48. La3+ ions act charge carrier trapping centers on the surface of TiO2, which suppresses the recombination of photogenerated electron-hole pairs and prolongs the lifetime, thus boosts the photocatalytic activity23,49. On the other hand, as the radius of Sn4+ ion is close to Ti4+ ion, Sn4+ ions are likely to substitute Ti4+ ions in the lattice of TiO2 and causes lattice distortion, which creates more surface defects. The surface defects are beneficial for the formation of oxygen vacancies and generating more superoxide radicals which degrade RhB molecules effectively20,27,28. Compared to pure and single element doped TiO2, Sn/La-TiO2 shows the highest photocatalytic activities at three temperatures because both Sn and La could enhance the photocatalytic activity as discussed before and Sn-La co-doping produces a synergistic effect. Moreover, from the results of XPS analysis, it is clear that the surface hydroxyl groups increase via Sn, La co-doping, which is propitious to promote photocatalytic activity. Besides, more surface area caused by Sn, La co-doping is also in favor of the enhancement of photocatalytic activity23,27.
Figure 11 presents the kinetic fitting plots of ln (C/C0) against irradiation time t for the photocatalysts, which show linear relationships, indicating that the degradation of RhB by TiO2 photocatalysts follows pseudo first order kinetics41. The apparent reaction rate constant k is calculated by:
where t is the reaction time, C and C0 are the concentration at time t and the initial concentration of RhB solution, respectively.
The ultimate degradation rates after 180 min and the apparent reaction rate constant k of all the photocatalysts are shown in Fig. 12 for a better comparison. The Sn/La-TiO2 also exhibit the fastest reaction rate and the apparent reaction rate constant k are 0.022 min−1, 0.027 min−1 and 0.024 min−1 at 360 °C, 450 °C and 600 °C, which are 3.7 times, 2.7 times and 8 times more than that of pure TiO2. The photocatalytic performance of TiO2 can be enhanced remarkably by Sn and La co-doping and the most noticeable enhancement effect occurs at the heat treatment temperature of 600 °C. The degradation data for all of the catalysts are shown in Table 2.
In order to study the effect of catalyst dosage, temperature, pH value and recyclability on the photocatalytic activity of catalyst, pure TiO2 annealed at 450 °C was chosen for the experiments.
In Fig. 13(a), the comparison of the degradation rate obtained with 0.25 g/L, 0. 5 g/L, 1 g/L, 1.5 g/L and 2 g/L catalyst is shown. The degradation rate increases with raising catalyst amount and the enhancement of degradation rate can be attributed to more surface area50. However, the degradation rate increases slightly when the concentration of catalyst surpasses 1 g/L. The aggregation of TiO2 particles occurs when the catalyst dosage is at high level, which leads to a decrease in surface area51.
The effect of temperature on the degradation rate of catalyst was studied by performed the photocatalytic experiments under temperatures ranging from 20 °C to 80 °C and the results are shown in Fig. 13(b). The degradation rare first increases when the temperature reach 60 °C and then decreases when the temperature is up to 80 °C. The oxidation rate of RhB molecules enhances with increase in temperature50. However, more rising the temperature is harmful to the photocatalytic activity because the recombination rate of photogenerated pairs and the desorption process of RhB molecules increase51.
In order to study the impact of the pH value on the degradation of RhB by catalyst, RhB solution was adjusted to different pH value (1, 4, 7, 10 and 13) by HCl (1 mol/L) and NaOH (1 mol/L). The results in Fig. 13(c) show that the degradation rate can be changed by varying the pH value. Acid environment shows higher degradation rate than neutral and alkaline environment. The PZC of pure TiO2 is about 6.25, therefore, the adsorption on catalyst is poor in alkaline solution. Acid solution is beneficial for reducing agglomeration and the formation of hydroxyl radicals, which lead to higher photocatalytic activity50.
The photocatalytic experiment was repeated four cycles to test the reusability of pure TiO2 catalyst and the results are shown in Fig. 13(d). The degradation rate after four cycles is 76.0%, which is slightly lower than that of the first cycle (82.5%). The decrease in the degradation rate can be attributed to the loss in adsorption between TiO2 catalyst and RhB molecules44.
The schematic diagram of band structure and electron pairs separation in Sn/La-TiO2 is shown in Fig. 14. The electrons in valence band (VB) are excited to conduction band (CB) when TiO2 catalyst is irradiated by UV light, leaving holes in valence band52. On the one hand, La3+ ions which on the surface of TiO2 can trap the photogenerated electrons in conduction band, consequently, the recombination of electrons and pairs is retarded and the photogenerated pairs are separated effectively47. On the other hand, the photogenerated electrons in conduction band of TiO2 can be transferred to conduction band of SnO2, since the conduction band energy level of SnO2 is lower than that of TiO219,53,54. Both Sn and La species are beneficial for the separation of photogenerated pairs, therefore, more electrons and holes can be involved into the photocatalytic degradation of RbB molecules. The holes combine with hydroxyl ions (OH−) to form hydroxyl radicals (.OH) and the electrons combine with adsorbed oxygen (O2) to form super-oxygen ions (.O2−)3,54. The resulting radicals degrade RbB molecules into smaller hydrocarbons and finally to CO2 and H2O55.
Conclusions
In summary, pure TiO2, Sn-TiO2, La-TiO2 and Sn/La-TiO2 nanomaterials experienced different temperature heat treatment were successfully synthesized via sol-gel route. The photocatalytic activity of the prepared TiO2 photocatalysts under UV light were tested by the degradation of RhB. Calcination temperature has an obvious influence on the photocatalytic property of pure TiO2 and it shows the best degradation rate at 450 °C. Doping with Sn and La enhances the photocatalytic activity of TiO2 significantly at three temperatures and Sn/La-TiO2 exhibit the highest degradation rates. The effect of co-doping on the improvement of photocatalytic performance can be attributed to the synergistic effect in suppressing photogenerated pairs and the formation of extra surface hydroxyl groups and more surface area involved during the reaction process.
References
Mishra, Y. K. & Adelung, R. ZnO tetrapod materials for functional applications. Materials Today. https://doi.org/10.1016/j.mattod.2017.11.003.
Jin, Y. et al. Hollow Znx Cd1−x S nanospheres with enhanced photocatalytic activity under visible light. Scientific Reports. 6, 2997–3004 (2016).
Tripathi, K. M., Tran, T. S., Kim, Y. J. & Kim, T. Y. Green fluorescent onion-like carbon nanoparticles from flaxseed oil for visible light induced photocatalytic applications and label-free detection of Al(III) ions. ACS Sustainable Chemistry & Engineering. 5, 3982–3992 (2017).
Mena, E., Rey, A., Rodríguez, E. M. & Beltrán, F. J. Reaction mechanism and kinetics of DEET visible light assisted photocatalytic ozonation with WO3 catalyst. Applied Catalysis B: Environmental. 202, 460–472 (2017).
Sahnesarayi, M. K., Sarpoolaky, H. & Rastegari, S. Effect of heat treatment temperature on the performance of nano-TiO2 coating in protecting 316L stainless steel against corrosion under UV illumination and dark conditions. Surface & Coatings Technology. 258, 861–870 (2014).
Shaban, M., Ashraf, A. M. & Abukhadra, M. R. TiO2 Nanoribbons/carbon nanotubes composite with enhanced photocatalytic activity; fabrication, characterization, and application. Scientific Reports. 8, 781–798 (2018).
Li, L. H. et al. The effects of Cu-doped TiO2 thin films on hyperplasia, inflammation and bacteria infection. Applied Sciences. 5, 1016–032 (2015).
Shang, J., Xu, W. W., Ye, C. X., George, C. & Zhu, T. Synergistic effect of nitrate-doped TiO2 aerosols on the fast photochemical oxidation of formaldehyde. Scientific Reports. 7, 1–9 (2017).
Iwasaki, M., Hara, M., Kawada, H., Tada, H. & Ito, S. Cobalt ion-doped TiO2 photocatalyst response to visible light. Journal of Colloid and Interface Science. 224, 202–204 (2000).
Lin, H. J., Yang, T. S., Hsi, C. S., Wang, M. C. & Lee, K. C. Optical and photocatalytic properties of Fe3+-doped TiO2 thin films prepared by a sol-gel spin coating. Ceramics International. 40, 10633–10640 (2014).
Demirci, S. et al. Synthesis and characterization of Ag doped TiO2 heterojunction films and their photocatalytic performances. Applied Surface Science. 390, 591–601 (2016).
Deng, X. Q., Liu, J. L., Li, X. S., Zhu, B. & Zhu, X. B. Kinetic study on visible-light photocatalytic removal of formaldehydefrom air over plasmonic Au/TiO2. Catalysis Today. 281, 630–635 (2017).
Yang, Z. L., Lu, J., Ye, W. C., Yu, C. S. & Chang, Y. L. Preparation of Pt/TiO2 hollow nanofibers with highly visible light photocatalytic activity. Applied Surface Science. 392, 472–480 (2017).
Liu, Y., Xie, C. S., Li, J., Zou, T. & Zeng, D. New insights into the relationship between photocatalytic activity and photocurrent of TiO2/WO3 nanocomposite. Applied Catalysis A: General. 433–434, 81–87 (2012).
El-Lateef, H. M. A. & Khalaf, M. M. Corrosion resistance of ZrO2-TiO2 nanocomposite multilayer thin films coated on carbon steel in hydrochloric acid solution. Materials Characterization. 108, 29–41 (2015).
Li, J., Lin, C. J., Li, J. T. & Lin, Z. Q. A photoelectrochemical study of CdS modified TiO2 nanotube arrays as photoanodes for cathodic protection of stainless steel. Thin Solid Films. 519, 5494–5502 (2011).
Zhu, Y. F. et al. Fabrication of heterostructured SrTiO3/TiO2 nanotube array films and their use in photocathodic protection of stainless steel. Electrochimica Acta. 121, 361–368 (2014).
Mazur, M., Morgiel, J., Wojcieszak, D., Kaczmarek, D. & Kalisz, M. Effect of Nd doping on structure and improvement of the properties of TiO2 thin films. Surface & Coatings Technology. 270, 57–65 (2015).
Du, J. M. et al. Synthesis and enhanced photocatalytic activity of black porous Zr-doped TiO2 monoliths. Nano. 11, 1650068–1650075 (2016).
Mohamed, R. M. & Aazam, E. S. Effect of Sn loading on the photocatalytic aniline synthesis activity of TiO2 nanospheres. Journal of Alloys and Compounds. 595, 8–13 (2014).
Wang, W., Liu, Y., Qu, J. F., Chen, Y. B. & Shao, Z. P. Nitrogen-doped TiO2 microspheres with hierarchical micro/nanostructures and rich dualphase junctions for enhanced photocatalytic Activity. RSC Advances. 6, 40923–40931 (2016).
Bakar, S. A. & Ribeiro, C. Rapid and morphology controlled synthesis of anionic S-doped TiO2 photocatalysts for visible-light-driven photodegradation of organic pollutants. RSC Advances. 6, 36516–36527 (2016).
Nešić, J. et al. Preparation, characterization and photocatalytic activity of lanthanum and vanadium co-doped mesoporous TiO2 for azo-dye degradation. Journal of Molecular Catalysis A: Chemical. 378, 67–75 (2013).
Peng, H., Cui, J., Zhan, H. J. & Zhang, X. Improved photodegradation and detoxification of 2,4,6-trichlorophenol by lanthanum doped magnetic TiO2. Chemical Engineering Journal. 264, 316–321 (2015).
Xin, Y. J. & Liu, H. L. Study on mechanism of photocatalytic performance of La-doped TiO2/Ti photoelectrodes by theoretical and experimental methods. Journal of Solid State Chemistry. 184, 3240–3246 (2011).
Solís-Casados, D. A., Escobar-Alarcón, L., Gómez-Oliván, L. M., Haro-Poniatowski, E. & Klimova, T. Photodegradation of pharmaceutical drugs using Sn-modified TiO2 powders under visible light irradiation. Fuel. 198, 3–10 (2017).
Li, J. L. et al. Sn doped TiO2 nanotube with oxygen vacancy for highly efficient visible light photocatalysis. Journal of Alloys and Compounds. 679, 454–462 (2016).
Bhange, P. D., Awate, S. V., Gholap, R. S., Gokavi, G. S. & Bhange, D. S. Photocatalytic degradation of methylene blue on Sn-doped titania nanoparticles synthesized by solution combustion route. Materials Research Bulletin. 76, 264–272 (2016).
Zhang, P. F., Li, X. W., Wu, X. K., Zhao, T. X. & Wen, L. S. Influence of In3+-doping and Ag0 -depositing on the visible-light-induced photocatalytic activity of TiO2. Journal of Alloys and Compounds. 673, 405–410 (2016).
Zhang, W. et al. Preparation, characterization, and photocatalytic activity of boron and lanthanum co-doped TiO2. Catalysis Communications. 45, 144–147 (2014).
Tripathi, A. K. et al. Structural, optical and photoconductivity of Sn and Mn doped TiO2 Nanoparticles. Journal of Alloys and Compounds. 622, 37–47 (2015).
Zhang, H. et al. Ni2+ and Ti3+ co-doped porous black anatase TiO2 with unprecedented-high visible-light-driven photocatalytic degradation performance. RSC Advances. 5, 107150–107157 (2015).
Naraginti, S. et al. Enhanced photo-catalytic activity of Sr and Ag co-doped TiO2 nanoparticles for the degradation of Direct Green-6 and Reactive Blue-160 under UV & visible light. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 149, 571–579 (2015).
Huang, T. Z. et al. Effects of N and F doping on structure and photocatalytic properties of anatase TiO2 nanoparticles. RSC Advances. 37, 16657–16664 (2013).
Lei, X. F., Xue, X. X. & Yang, H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr(VI) under visible light. Applied Surface Science. 321, 396–403 (2014).
Ding, X. Z., Liu, L., Ma, X. M., Qi, Z. Z. & He, Y. Z. The influence of alumina dopant on the structural transformation of gel-derived nanometre titania powders. Journal of Materials Science Letters. 13, 462–464 (1994).
Spurr, R. A. & Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-Ray diffractometer. Analytical Chemistry. 29, 760–762 (1957).
Uvarov, V. & Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Materials Characterization. 58, 883–891 (2007).
Dou, L. et al. P123-assisted hydrothermal synthesis of BiOI with enhanced photocatalytic performance. Materials Letters. 153, 179–181 (2016).
Begum, A., Tripathi, K. M. & Sarkar, S. Water-induced formation, characterization, and photoluminescence of carbon nanotube-based composites of Gadolinium(III) and Platinum(II) dithiolenes. Chemistry-A European Journal. 20, 1–6 (2014).
Zhang, Y., Wang, T., Zhou, M., Wang, Y. & Zhang, Z. M. Hydrothermal preparation of Ag-TiO2 nanostructures with exposed {001}/{101} facets for enhancing visible light photocatalytic activity. Ceramics International. 43, 3118–3126 (2017).
Huang, D. G. et al. Synthesis of samarium- and nitrogen-co-doped TiO2 by modified hydrothermal method and its photocatalytic performance for the degradation of 4-chlorophenol. Journal of Physics and Chemistry of Solids. 70, 853–859 (2009).
Yu, Y. M. et al. A facile one-pot synthesis of N-La codoped TiO2 porous materials with bio-hierarchical architectures and enhanced photocatalytic activity. Materials Chemistry and Physics. 182, 77–85 (2016).
Vaiano, V., Sacco, O., Sannino, D. & Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Applied Catalysis B: Environmental. 170–171, 153–161 (2015).
Tang, X. K., Feng, Q. M., Lin, K. & Tan, Y. Synthesis and characterization of a novel nanofibrous TiO2/SiO2 composite with enhanced photocatalytic activity. Materials Letters. 183, 175–178 (2016).
Alves, A. K., Berutti, F. A. & Bergmann, C. P. Visible and UV photocatalytic characterization of Sn-TiO2 electrospun fibers. Catalysis Today. 208, 7–10 (2013).
Ilkhechi, N. N., Akbarpour, M. R., Yavari, R. & Azar, Z. Sn4+ and La3+ co doped TiO2 nanoparticles and their optical, photocatalytic and antibacterial properties under visible light. Journal of Materials Science Materials in Electronics. 28, 16658–16664 (2017).
Bokare, A., Pai, M. & Athawale, A. A. Surface modified Nd doped TiO2 nanoparticles as photocatalysts in UV and solar light irradiation. Solar Energy. 91, 111–119 (2013).
Du, J. M. et al. A facile sol-gel method for synthesis of porous Nd-doped TiO2 monolith with enhanced photocatalytic activity under UV-Vis irradiation. Microporous and Mesoporous Materials. 182, 87–94 (2013).
Pazoki, M., Parsa, M. & Farhadpour, R. Removal of the hormones dexamethasone (DXM) by Ag doped on TiO2 photocatalysis. Journal of Environmental Chemical Engineering. 4, 4426–4434 (2016).
Malik, R. et al. Nano gold supported on ordered mesoporous WO3/SBA-15 hybrid nanocomposite for oxidative decolorization of azo dye. Microporous and Mesoporous Materials. 225, 245–254 (2016).
Wang, T. et al. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Physica E. 86, 103–110 (2017).
Tomer, V. K. & Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. Journal of Materials Chemistry A. 4, 1033–1043 (2016).
Kadam, A., Dhabbe, R., Shin, D. S., Garadkar, K. & Park, J. Sunlight driven high photocatalytic activity of Sn doped N-TiO2 nanoparticles synthesized by a microwave assisted method. Ceramics International. 43, 5164–5172 (2017).
Khare, P. et al. Sunlight-induced selective photocatalytic degradation of methylene blue in bacterial culture by pollutant soot derived nontoxic graphene nanosheets. ACS Sustainable Chemistry & Engineering. 6, 579–589 (2018).
Acknowledgements
This work was supported by the Open Research Subject of Powder Metallurgy Engineering Technology Research Center of Sichuan Province, China (grant no. SC-FMYJ2017-03, SC-FMYJ2017-05), the Applied Basic Research Programs of Sichuan province, China (grant no. 2018JY0062), the Soft Science Project of Chengdu, China (grant no. 2016-RK00-00044-ZF), the National Natural Science Research Foundation of China (grant no. 11572057), the Open Research Subject of Key Laboratory of Special Material and Preparation Technology of Sichuan Province, China (grant no. szjj2017-062), the Training Program for Innovation of Chengdu University, China (grant no. CDU-CX-2018001, CDU-CX-2018003).
Author information
Authors and Affiliations
Contributions
X.D. Zhu and W. Feng designed the experiments, characterized the samples, analyzed the data, and wrote the manuscript. L.X. Pei and R.R. Zhu carried out the specific experimental process and analyzed the data. Y. Jiao carried out TEM tests and photocatalytic tests according to the suggestions of reviewers. R.Y. Tang contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, X., Pei, L., Zhu, R. et al. Preparation and characterization of Sn/La co-doped TiO2 nanomaterials and their phase transformation and photocatalytic activity. Sci Rep 8, 12387 (2018). https://doi.org/10.1038/s41598-018-30050-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30050-3
- Springer Nature Limited
This article is cited by
-
Comparative Toxicity of TiO2 and Sn-Doped TiO2 Nanoparticles in Zebrafish After Acute and Chronic Exposure
Biological Trace Element Research (2024)
-
Fabrication of Al and La co-doped CdS thin film for ammonia gas-sensing application through low-cost nebulizer spray pyrolysis technique
Applied Physics A (2024)
-
Understanding the doping mechanism of Sn in TiO2 nanorods toward efficient photoelectrochemical performance
Journal of Materials Science (2023)
-
Effect of Both Sn Doping and Annealing Temperature on the Properties of Dip-Coated Nanostructured TiO2 Thin Films
Journal of Inorganic and Organometallic Polymers and Materials (2022)
-
Synergic effect of Sn-doped TiO2 nanostructures for enhanced visible light photocatalysis
Journal of Materials Science: Materials in Electronics (2022)