Abstract
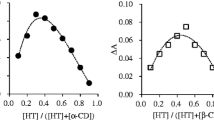
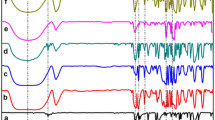
Molecular inclusion complexes of usnic acid (UA) with β-cyclodextrin (β-CD) and 2-hydroxypropyl β-cyclodextrin (HP β-CD) were prepared by the co-precipitation method in the solid state in the molar ratio of 1:1. Structural complexes characterization was based on different methods, FTIR, 1H NMR, XRD and DSC. Parallel to the complex by the above methods, corresponding physical mixtures of UA with cyclodextrins and complexing agents (β-CD, HP β-CD and UA) were analyzed. The results of DSC analysis showed that, at around 200 °C, the endothermal peak in the complexes with cyclodextrins originating from the UA melting has disappeared. Complex diffractogram patterns do not contain peaks characteristic for the pure UA. They are more appropriate to cyclodextrin diffractogram. This fact points to the molecular encapsulation of UA in the cyclodextrin cavity. Chemical shifts in 1H NMR spectra after the inclusion of UA into the cyclodextrin cavity, especially H-3 protons (0.0012 and 0.0102 ppm in the β-CD and HP β-CD, respectively) and H-5 and H-6 (0.0134 ppm) and hydrogen from CH3 (0.0073 ppm) HP β-CD also points to the formation of molecular inclusion complexes. The improved solubility of UA in water was achieved by molecular incapsulation. In the complex with β-CD the solubility is 0.3 mg/cm3, with HP β-CD 4.2 mg/cm3 while the uncomplexed UA solubility is 0.06 mg/cm3. The microbial activity of UA and both complexes was tested against eight bacteria and two fungi and during the test no reduced activity of UA in the complexes was observed.







Similar content being viewed by others
References
Vartia, K.O.: Antibiotics in lichens. In: Ahmadjian, V., Hale, M.E. (eds.) The lichens, pp. 547–561. Academic Press, New York (1973)
Proska, B., Sturdikova, M., Pronayova, N., Liptaj, T.: Usnic acid and its derivatives. Pharmazie 51, 195–196 (1996)
Wu, J., Zhang, M., Ding, D., Tan, T., Yan, B.: Effect of CIadoni a alpestris on Trichmonas in vitro. Chinese J. Parasit. Dis. 13, 126–129 (1995)
Perry, N., Benn, M., Brennan, N., Burgess, E., Ellis, G., Galloway, D., Lorimer, S., Tangney, R.: Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist 31, 627–636 (1999)
Yamamoto, Y., Miura, Y., Kinoshita, Y., Higuchi, C.M., Yamada, Y., Murakami, A., Ohigashi, H., Koshimizu, K.: Screening of tissue cultures and thalli of lichens and some of their active constituents for inhibition of tumor promoter-induced Epstein-Barr virus activation. Chem. Pharm. Bull. 43(8), 1388–1477 (1995)
Takai, M., Uehara, Y., Beisler, J.: Usnic acid derivatives as potential antineoplastic agents. J. Med. Chem. 22, 1380–1384 (1979)
Kristmundsdottir, T., Aradottir, R., Ingolfsdottir, K., Ogmundsdottir, R.: Solubilization of the lichen metabolite usnic acid for testing in tissue culture. J. Pharm. Pharmacol. 54(11), 1447–1452 (2002)
Vijayakumar, C., Viswanathan, S., Kannappa-Reddy, M., Parvathavarthini, S., Kundu, S., Sukumar, E.: Anti-inflammatory activity of (+)-usnic acid. Fitoterapia 71, 564–566 (2000)
Okuyama, E., Umeyama, K., Yamazaki, M., Kinoshita, Y., Yamamoto, Y.: Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 61(2), 113–118 (1995)
Stoll, A., Brack, A., Renz, J.: Die wirkung von flectenstoffen auf tubefkelbaktericn-unf auf einigen andere mokroorganismen. Schweiz Z. Path. Bakt. 13, 729–751 (1950)
Ingolfsdottir, K.: Usnic acid. Phytochemistry 61, 729–736 (2002)
Konschai, U.: Antiseptische lokaltherapeutika als eigenstanddige therapie NHO-arztlich gesehen. Therapiewoche 27, 7732–7735 (1977)
Wunderer, H.: Mund- und Rachentherapeutika. D. Apoth. Ztg. 126, 2281–2292 (1986)
Seifetr, P., Bertram, C.: Usnic acid natural-preservation from lichens. Seifen Ole Fette Wachse 121, 480–485 (1995)
Nikolić, G., Zlatković, S., Kundaković, T., Đokić, D., Đorđević, A., Savić, I.: Study and purpose of usnic acid mother liquor as potential antiseptic in some products. J. Int. Sci. Publ. Mater. Methods Technol. 4(1), 294–310 (2010)
Huneck, S., Yoshimura, Y.: Identification of Lichen Substances. Springer, New York (1996)
Ingolfsdottir, K., Chung, G., Skulason, V., Gissurarson, S., Vilhelmsdottir, M.: In vitro antimycobacterial activity of lichen metabolites. Eur. J. Pharm. Sci. 6, 141–144 (1998)
Cakić, M., Nikolić, G., Nikolić, N., Stanković, M.: FT-IR spektri derivata natrijumove soli usninske kiseline i njegovih deuterisanih analoga, III Symposium: new technologies and economic development, p. 74. Faculty of Technology, Leskovac (1998)
Stanković, M., Stanković, S., Djordjević, Z., Djordjević, S.: Postupak dobijanja natrijumove soli (+)-usninske kiseline. RS 43978, P-134/86 (1989)
Stanković, S., Djordjević, Z., Stanković, M., Djordjević, S.: Postupak za dobijanje natrijumove soli (+)-usninske kiseline. RS-2061/88 (1988)
Stankovic, M., Randjelovic, M.: Postupak za izolaciju (+)-usninske kiseline iz Usnea barbata L. RS 42564, P-919/81 (1981)
Stanković, S., Djordjevic, I., Kocić, Z.: Pharmaceutical disinfectants comprising usnic acid and essential oils. EP 1294373 (2001)
Sladić, D., Beljanski, V., Prelesnik, B., Bogdanović, G., Ivanović, I., Andjelkovic, K.: Preparation, crystal structure and antibacterial activity of condensation products of usnic acid and acyl hydrazides. J. Serb. Chem. Soc. 63(3), 171–182 (1998)
Nikolić, G., Zlatković, S., Nikolić, N.: Identification and compatibility of the major active principles in some new natural origin antiseptics. Russ. J. Phys. Chem. A 83(9), 1612–1616 (2009)
Nikolić, V., Nikolić, Lj., Stanković, M., Kapor, A., Popsavin, M., Cvetković, D.: A molecular inclusion complex of atenolol with 2-hydroxypropyl-β-cyclodextrin; the production and characterization thereof. J. Serb. Chem. Soc. 72(8–9), 737–746 (2007)
Ficarra, R., Ficarra, P., Di Bella, M.R., Raneri, D., Tommasini, S., Calabro, M.L., Gamberini, M.C., Rustichelli, C.: Study of β-blockers/β-cyclodextrins inclusion complex by NMR, DSC, X-ray and SEM investigation. J. Pharm. Biomed. Anal. 23, 33–40 (2000)
Ficarra, R., Ficarra, P., Di Bella, M.R., Raneri, D., Tommasini, S., Calabro, M.L., Villari, A., Coppolino, S.: Study of the inclusion complex of atenolol with β-cyclodextrins. J. Pharm. Biomed. Anal. 23, 232–236 (2000)
Nikolić, V., Ilić, D., Nikolić, Lj., Stanković, M., Cakić, M., Stanojević, Lj., Kapor, A., Popsavin, M.: The protection of Nifedipin from photodegradation due to complex formation with β-cyclodextrin. Cent. Eur. J. Chem. 8(4), 744–749 (2010)
Mielcarek, J.: Photochemical stability of the inclusion complexes of nicardipine with α-, γ-cyclodextrin methyl-β-cyclodextrin, hydroxypropyl-β-cyclodextrins in the solid state and in solution. Pharmazie 51, 477–479 (1996)
Chan, K.L.A., Kazarian, S.G.: FTIR spectroscopic imaging of dissolution of a solid dispersion of nifedipine in poly(ethylene glycol). Mol. Pharm. 1, 331–335 (2004)
Kapor, A., Nikolić, V., Nikolić, Lj., Stanković, M., Cakić, M., Stanojević, Lj., Ilić, D.: Inclusion complexes of amlodipine besylate and cyclodextrins. Cent. Eur. J. Chem. 8(4), 834–841 (2010)
Mielcarek, J., Czernielewska, A., Czarczynska, B.: Inclusion complexes of felodipine and amlodipine with methyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 54, 17–21 (2006)
Nikolić, V., Stanković, M., Kapor, A., Nikolić, Lj., Cvetković, D., Stamenković, J.: Allylthiosulfinate; β-cyclodextrin inclusion complex: preparation, characterization and microbiological activity. Pharmazie 59, 845–848 (2004)
Lira, M.C.B., Ferraz, M.S., da Silva, D.G.V.C., Cortes, M.E., Teixeria, K.I., Caetano, N.P., Sinisterra, R.D., ponchel, G., Santos-magalhes, N.S.: Inclusion complex of usnic acid with b-cyclodextrin: characterization and nanoencapsulation into liposomes. J. Incl. Phenom. Macrocycl. Chem. 64, 215–224 (2009)
Cocchietto, M., Skert, N., Nimis, P.L., Sava, G.: A review on usnic acid, an interesting natural compound. Naturwissenchaften 89, 137–146 (2002)
Ghione, M., Parrello, D., Grasso, L.: Usnic acid revisited its activity on oral flora. Chemoterapia 7, 302–305 (1988)
Lauterwein, M., Oethinger, M., Belsner, K., Peters, T., Marre, R.: In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid and (-)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother. 39, 2541–2543 (1995)
Ingólfsdóttir, K.: Usnic acid. Phytochemistry 61, 729–736 (2002)
Acknowledgments
This work is a part of the research done within the project TR 34012. The authors would like to thank the Ministry of Education and Science, Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikolić, V., Stanković, M., Nikolić, L. et al. Inclusion complexes with cyclodextrin and usnic acid. J Incl Phenom Macrocycl Chem 76, 173–182 (2013). https://doi.org/10.1007/s10847-012-0187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0187-8