Abstract
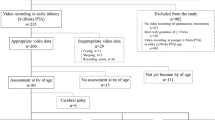
Gross motor development (supine, prone, rolling, sitting, crawling, walking) and movement abnormalities were examined in the home videos of infants later diagnosed with autism (regression and no regression subgroups), developmental delays (DD), or typical development. Group differences in maturity were found for walking, prone, and supine, with the DD and Autism-No Regression groups both showing later developing motor maturity than typical children. The only statistically significant differences in movement abnormalities were in the DD group; the two autism groups did not differ from the typical group in rates of movement abnormalities or lack of protective responses. These findings do not replicate previous investigations suggesting that early motor abnormalities seen on home video can assist in early identification of autism.

Similar content being viewed by others
Notes
Two forms of roll behavior were originally coded: roll supine-to-prone and roll prone-to-supine. The frequencies of both types of roll behavior, however, were too low to warrant separate analyses and therefore were collapsed into a single “roll” behavior.
We used child’s average age at each maturity level as the best representation of reliable performance of the respective motor behavior at any given maturity level. Although we also examined earliest age of maturity level appearance, the results were not substantially different than those presented for mean age. For subjects who did not have multiple instances of a behavior at a given maturity level, the data for that maturity level was not used in the analysis.
References
Adrien, J. L., Lenoir, P., Martineau, J., Perrot, A., Hameury, L., Larmande, C., et al. (1993). Blind ratings of early symptoms of autism based on family home movies. Journal of the American Academy of Child and Adolescent Psychiatry, 32, 617–626.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, 4th edition, text revision. Washington DC: Author.
Baranek, G. (1999). Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders, 29, 213–224.
Baranek, G. (2002). Efficacy of sensory and motor interventions for children with autism. Journal of Autism and Developmental Disorders, 32, 397–422.
Bryson, S. E., Zwaigenbaum, L., Brian, J., Roberts, W., Szatmari, P., Rombough, V., et al. (2007). A prospective case series of high-risk infants who develop autism. Journal of Autism and Developmental Disorders, 37, 12–24.
Damasio, A. R., & Maurer, R. G. (1978). A neurological model for childhood autism. Archives of Neurology, 35, 777–786.
Gillberg, C., Ehlers, S., Schaumann, H., Jakobsson, G., Dahlgren, S. O., Lindblom, R., et al. (1990). Autism under 3 years of age: A clinical study of 28 cases referred for autistic symptoms in infancy. Journal of Child Psychology and Psychiatry, 31, 921–934.
Goldberg, W. A., Osann, K., Flodman, P., Spence, M. A., Jarvis, K., Modahl, C., et al. (2003). Language and other regression: Assessment and timing. Journal of Autism and Developmental Disorders, 33, 607–616.
Hallett, M., Lebiedowska, M. K., Thomas, S. L., Stanhope, S. J., Denckla, M. B., & Rumsey, J. (1993). Locomotion of autistic adults. Archives of Neurology, 50, 1304–1308.
Jansiewicz, E. M., Goldberg, M. C., Newschaffer, C. J., Denckla, M. B., Landa, R., & Mostofsky, S. H. (2006). Motor signs distinguish children with high functioning autism and Asperger syndrome from controls. Journal of Autism and Developmental Disorders, 36, 613–621.
Landa, R., & Garrett-Mayer, E. (2006). Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry, 47, 629–638.
Lord, C., Rutter, M., DiLavore, P. C., & Risi, S. (2002). Autism diagnostic observation schedule manual. Los Angeles: Western Psychological Services.
Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685.
Mayes, S. D., & Calhoun, S. L. (2003). Ability profiles in children with autism: Influences of age and IQ. Autism, 7, 65–80.
Minshew, N. J., Sung, K., Jones, B. L., & Furman, J. M. (2004). Underdevelopment of the postural control system in autism. Neurology, 63, 2056–2061.
Miyahara, M., Tsujii, M., Hori, M., Nakanishi, K., Kageyama, H., & Sugiyama, T. (1997). Motor incoordination in children with Asperger syndrome and learning disabilities. Journal of Autism and Developmental Disorders, 27, 595–603.
Morin, B., & Reid, G. (1985). A quantitative and qualitative assessment of autistic individuals on selected motor tasks. Adapted Physical Activity Quarterly, 2, 43–55.
Mullen, E. M. (1995). Mullen scales of early learning. Circle Pines, MN: AGS.
Noterdaeme, M., Mildenberger, K., Minow, F., & Amorosa, H. (2002). Evaluation of neuromotor deficits in children with autism and children with a specific speech and language disorder. European Child and Adolescent Psychiatry, 11, 219–225.
Ozonoff, S., Williams, B. J., & Landa, R. (2005). Parental report of the early development of children with regressive autism: Delays-plus-regression phenotype. Autism, 9, 461–486.
Page, J., & Boucher, J. (1998). Motor impairments in children with autistic disorder. Child Language and Teaching Therapy, 14, 233–259.
Provost, B., Lopez, B. R., & Heimerl, S. (2006). A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders, 37, 321–328.
Rimland, B. (1964). Infantile autism: The syndrome and its implications for a neural theory of behavior. Englewood Cliffs NJ: Prentice-Hall.
Rogers, S. J., Hepburn, S. L., Stackhouse, T., & Wehner, E. (2003). Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry, 44, 763–781.
Sparrow, S. S., Balla, D. A., & Cicchetti, D. (1984). Vineland adaptive behavior scales. Circle Pines, MN: AGS.
Teitelbaum, O., Benton, T., Shah, P. V., Prince, A., Kelly, J. K., & Teitelbaum, P. (2004). Eshkol-Wachman movement notation in diagnosis: The early detection of Asperger syndrome. Proceedings of the National Academy of Sciences, 101, 11909–11914.
Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman, J., & Maurer, R. G. (1998). Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences, 95, 13982–13987.
Vilensky, J. A., Damasio, A. R., & Maurer, R. G. (1981). Gait disturbance in patients with autistic behavior: A preliminary study. Archives of Neurology, 38, 646–649.
Werner, E., Dawson, G., Munson, J., & Osterling, J. (2005). Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders, 35, 337–350.
Yirmiya, N., Gamliel, I., Pilowsky, T., Feldman, R., Baron-Cohen, S., & Sigman, M. (2006). The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology & Psychiatry, 47, 511–523.
Yirmiya, N., & Ozonoff, S. (2007). The very early phenotype of autism. Journal of Autism and Developmental Disorders, 37, 1–11.
Zwaigenbaum, L., Bryson, S., Rogers, T., Roberts, W., Brian, J., & Szatmari, P. (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23, 143–152.
Acknowledgments
The work in this manuscript was supported by grant U19 HD35468 from the National Institute of Child Health and Human Development. The authors would like to acknowledge Karim Bayanzay, Corinna Conway, Jackelynn Dao, Naomi Ekas, Jason Gray, Elizabeth Loomer, Matthew Ong, Angela Shih, and Christina Siller who coded the many hours of video presented here, as well as Diane Larzelere who prepared the manuscript. We are very grateful to Grace Baranek for sharing her content coding system with us. We are also greatly indebted to the many families who shared their home videotapes and the lives of their families with us.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozonoff, S., Young, G.S., Goldring, S. et al. Gross Motor Development, Movement Abnormalities, and Early Identification of Autism. J Autism Dev Disord 38, 644–656 (2008). https://doi.org/10.1007/s10803-007-0430-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-007-0430-0